Question: 11. Use the data in the table to answer the questions that follow for the reaction: BrO3(aq)+5Br(aq)+6H+(aq)3Br2(l)+3H2O(l) a) Determine the rate law. b) Calculate the
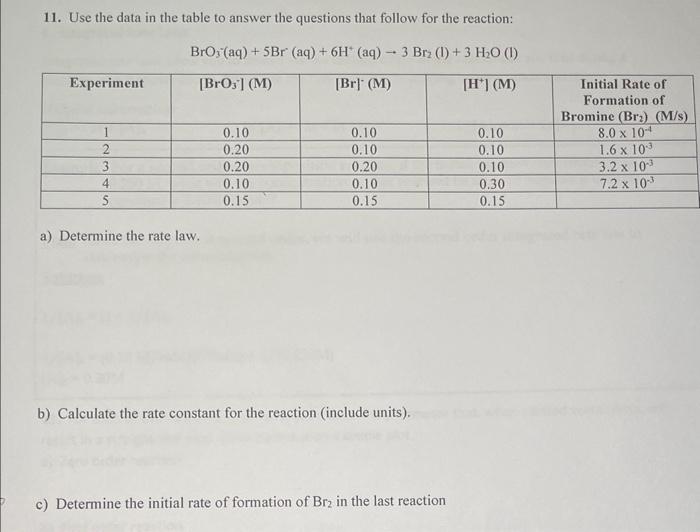
11. Use the data in the table to answer the questions that follow for the reaction: BrO3(aq)+5Br(aq)+6H+(aq)3Br2(l)+3H2O(l) a) Determine the rate law. b) Calculate the rate constant for the reaction (include units). c) Determine the initial rate of formation of Br2 in the last reaction
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


