Question: 11. You will be extensively studying aromatic compounds this semester. You will learn that some aromatic rings are more electrophilic than others. Draw all resonance
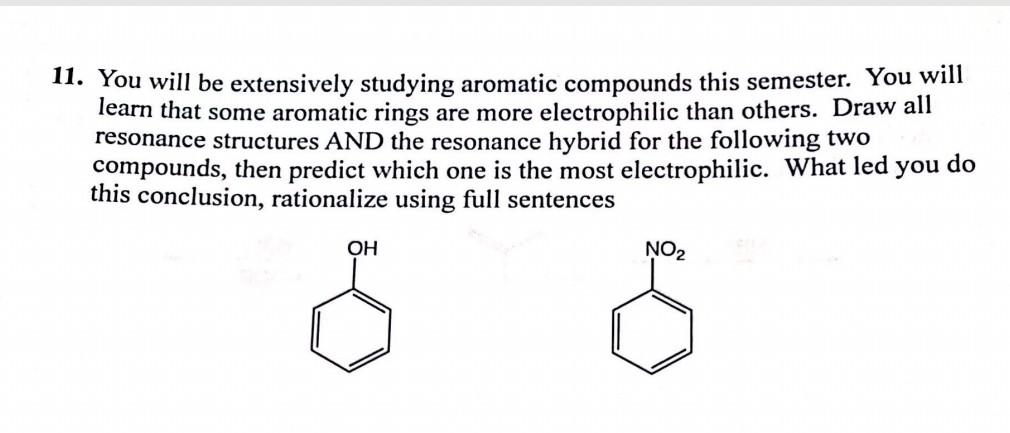
11. You will be extensively studying aromatic compounds this semester. You will learn that some aromatic rings are more electrophilic than others. Draw all resonance structures AND the resonance hybrid for the following two compounds, then predict which one is the most electrophilic. What led you do this conclusion, rationalize using full sentences OH NO2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


