Question: !!!!! 12 10 00 8 PH 0) 6 N 2. 4 - + 10 + 25 1 30 5 15 20 35 VOLUME OF NaOH
!!!!!
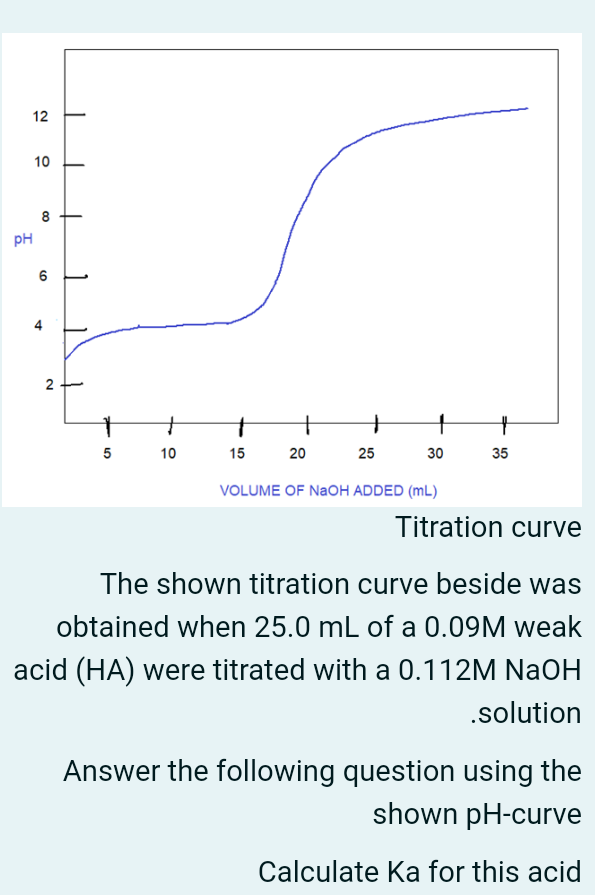
12 10 00 8 PH 0) 6 N 2. 4 - + 10 + 25 1 30 5 15 20 35 VOLUME OF NaOH ADDED (ML) Titration curve The shown titration curve beside was obtained when 25.0 mL of a 0.09M weak acid (HA) were titrated with a 0.112M NaOH .solution Answer the following question using the shown pH-curve Calculate Ka for this acid
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


