Question: 12. Using Le Chatelier's principles, for the following equilibrium predict the direction aid that the equilibrium will shift (towards the products or towards the reactants)
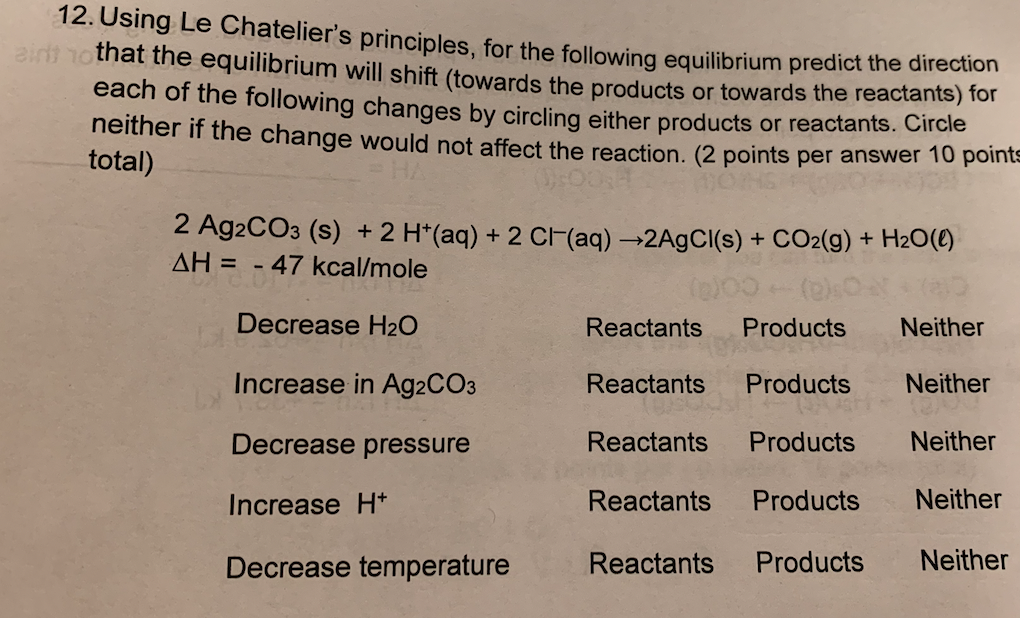
12. Using Le Chatelier's principles, for the following equilibrium predict the direction aid that the equilibrium will shift (towards the products or towards the reactants) for each of the following changes by circling either products or reactants. Circle neither if the change would not affect the reaction. (2 points per answer 10 points total) 2 Ag2CO3 (s) + 2 H+(aq) + 2 CF(aq) -2AgCl(s) + CO2(g) + H2O(C) AH = - 47 kcal/mole Decrease H2O Reactants Products Neither Increase in Ag2CO3 Reactants Products Neither Decrease pressure Reactants Products Neither Increase H+ Reactants Products Neither Decrease temperature Reactants Products Neither
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


