Question: 125. 4 2P +- 3 Pressure P+----= -- - -- 2V Volume A sample of an ideal monatomic gas can be taken through each of
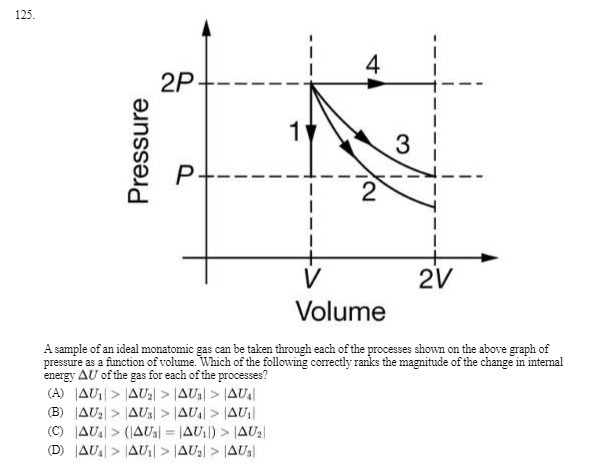
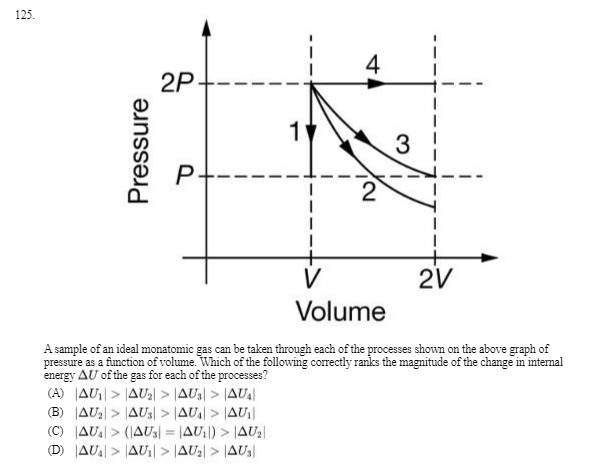
125. 4 2P +- 3 Pressure P+----= -- - -- 2V Volume A sample of an ideal monatomic gas can be taken through each of the processes shown on the above graph of pressure as a function of volume. Which of the following correctly ranks the magnitude of the change in internal energy AU of the gas for each of the processes? (A) [AU, > |4U, > |4U,) > |4U. (B) |AU, > |4Us) > |AU) > LAUII (C) AU.) > (14Us) = 14U1)) > 14U2 (D) |AU. > |4UI| > |4U.) > JAUs
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


