Question: 13. a (13.1) Derive a relationship between the equilibrium constant of a chemical reaction and the standard Gibbs energy change, A,G. (13.2) The dissociation constant,
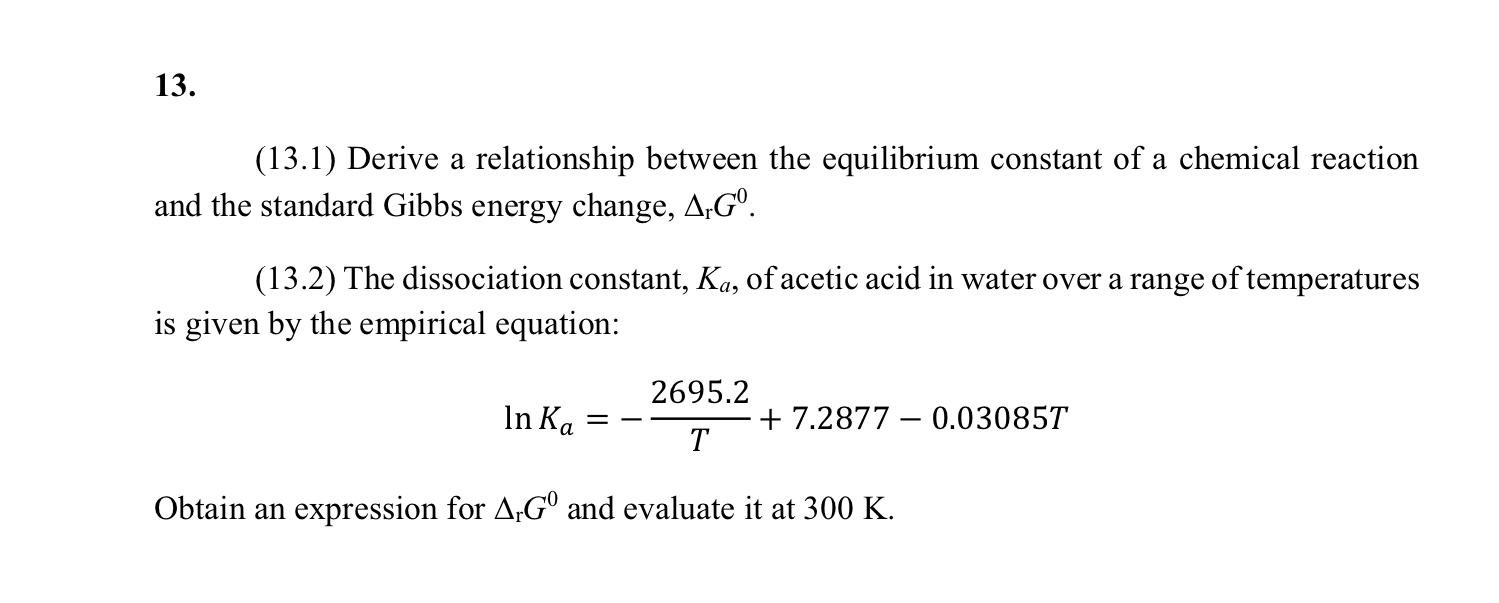
13. a (13.1) Derive a relationship between the equilibrium constant of a chemical reaction and the standard Gibbs energy change, A,G. (13.2) The dissociation constant, Ka, of acetic acid in water over a range of temperatures is given by the empirical equation: In Ka 2695.2 + 7.2877 0.03085T T - Obtain an expression for A G and evaluate it at 300 K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


