Question: 13. According to the following activity series: MgZnFePbH2Cu( decrease reactivity) Which of the following equations show no reactions? (I)Fe(s)+PbSO4(aq)(II)Cu(s)+ZnSO4(aq)(III)Fe(s)+CuSO4(aq)(IV)Zn(s)+2HClaq)(V)Pb(s)+MgSO4(aq) A) (I) only B) (II) only
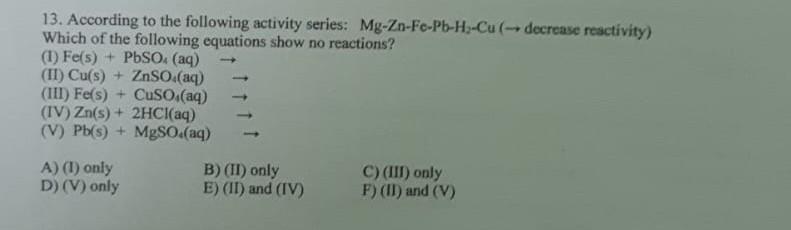
13. According to the following activity series: MgZnFePbH2Cu( decrease reactivity) Which of the following equations show no reactions? (I)Fe(s)+PbSO4(aq)(II)Cu(s)+ZnSO4(aq)(III)Fe(s)+CuSO4(aq)(IV)Zn(s)+2HClaq)(V)Pb(s)+MgSO4(aq) A) (I) only B) (II) only C) (III) only D) (V) only E) (II) and (IV) F) (II) and (V)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


