Question: 13 Question (1 point) Below is a reaction between water and borane. 2nd attempt Part 1(0.5 point) Which is the most electron poor atom in
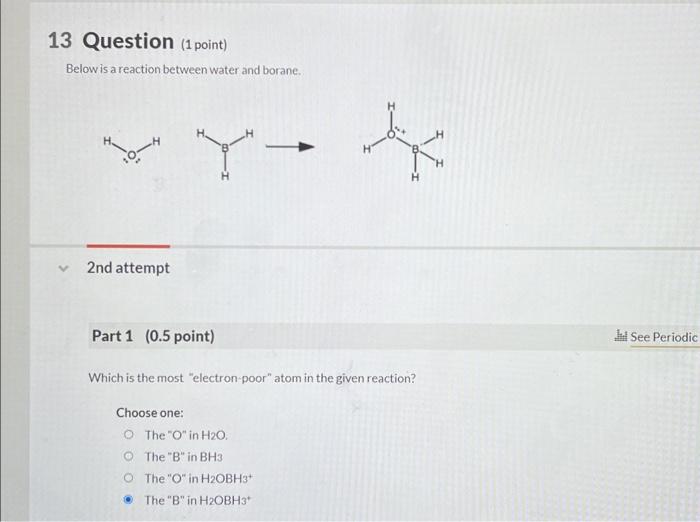
13 Question (1 point) Below is a reaction between water and borane. 2nd attempt Part 1(0.5 point) Which is the most "electron poor" atom in the given reaction? Choose one: The " O1 in H2O. The "B" in BH3 The "O" in H2OBH3+ The "B" in H2OBH3 + Select the elementary mechanisticstep: Choose one: nucleophilic elimination coordination electrophilic addition carbocation rearrangement
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


