Question: Below is a reaction between water and borane. 1st attempt Part 1 ( 0.5 point) Which is the most electron-poor atom in the given reaction?
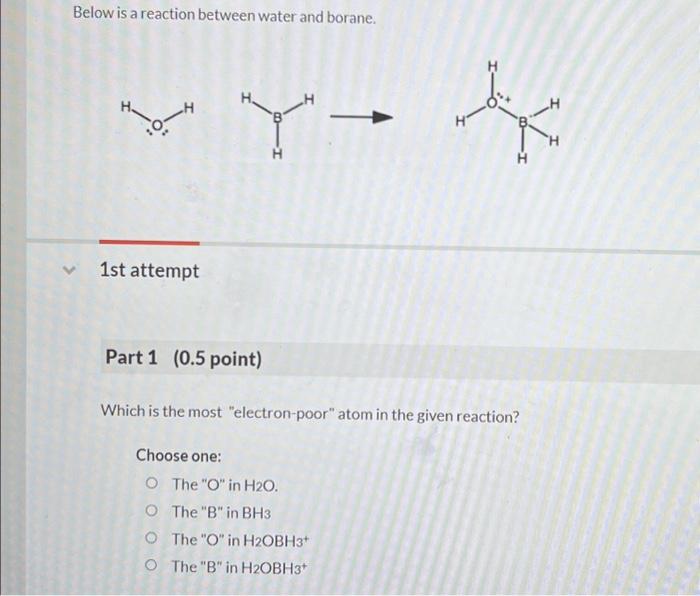
Below is a reaction between water and borane. 1st attempt Part 1 ( 0.5 point) Which is the most "electron-poor" atom in the given reaction? Choose one: The " O " in H2O. The "B" in BH3 The "O" in H2OBH3+ The " B " in H2OBH3+
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


