Question: 13 Question (1 point) The balanced equation for the combustion of butane is: 2C4H10+13O28CO2+1OH2O 1st attempt If the combustion of 56.09g of CAH1 produces 105.94g
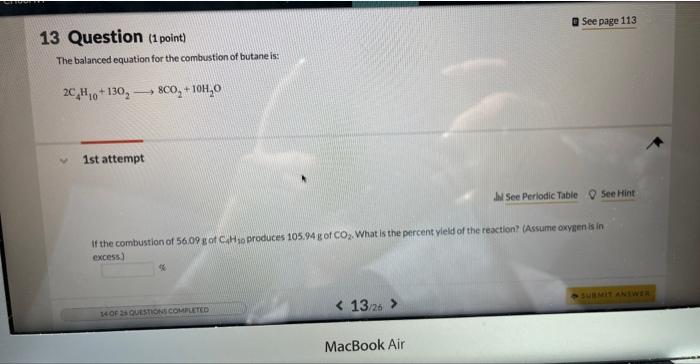
13 Question (1 point) The balanced equation for the combustion of butane is: 2C4H10+13O28CO2+1OH2O 1st attempt If the combustion of 56.09g of CAH1 produces 105.94g of CO2. What is the percent vield of the reaction? (Assume oxygen is in cxcess)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


