Question: 13 Question (2points) A Wrie ruby has a mass of 12.040 carats 11 carat = 20000 mp, Ribies are made of a crystalline form of
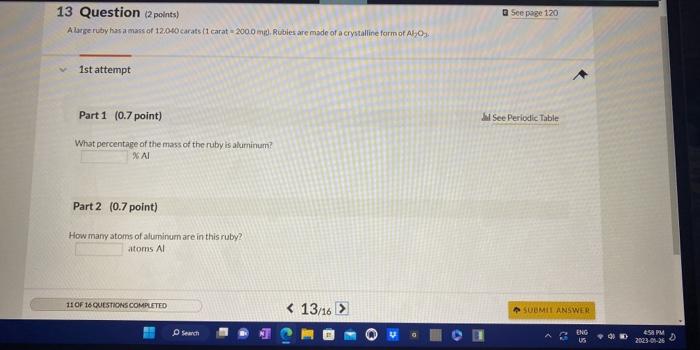
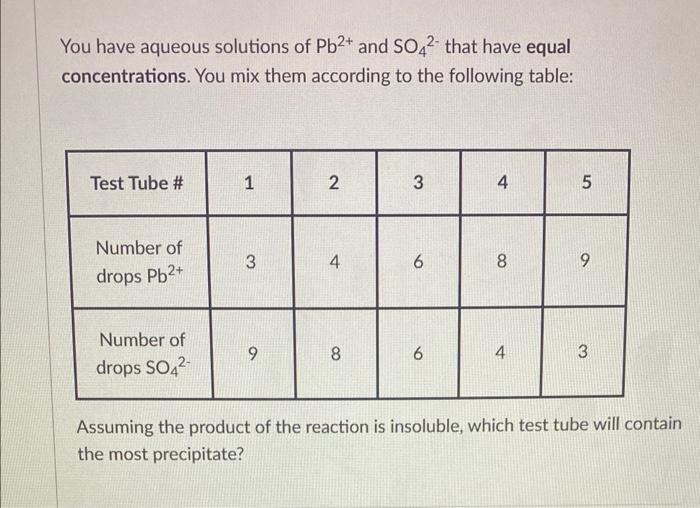
13 Question (2points) A Wrie ruby has a mass of 12.040 carats 11 carat = 20000 mp, Ribies are made of a crystalline form of A2Oy : 1st attempt Part 1 (0.7 point) What percentage of the mass of the ruby is aluminum? 26 Al Part 2 (0.7 point) Howinamy atoms of aluminum are in this ruby? atoms Al You have aqueous solutions of Pb2+ and SO42 that have equal concentrations. You mix them according to the following table: Assuming the product of the reaction is insoluble, which test tube will contain the most precipitate
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


