Question: please help (3.) This is a Numeric Entry question / It is worth 2 points / You have unlimited attempts/There is no attempt penalty 12
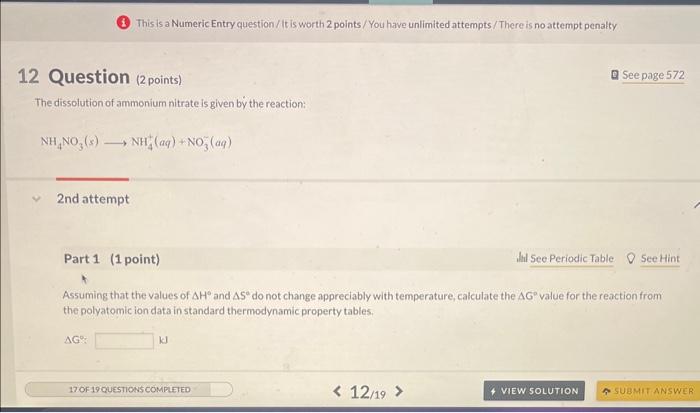
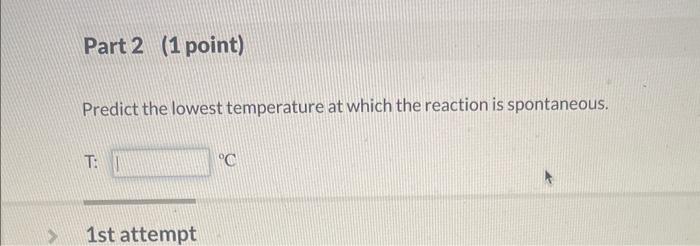
(3.) This is a Numeric Entry question / It is worth 2 points / You have unlimited attempts/There is no attempt penalty 12 Question (2points) Q See page 572 The dissolution of ammonium nitrate is given by the reaction: NH4NO3(s)NH4+(aq)+NO3(aq) 2nd attempt Part 1 (1 point) Assuming that the values of H and S do not change appreciably with temperature, calculate the G value for the reaction from the polyatomic ion data in standard thermodynamic property tables. G: Predict the lowest temperature at which the reaction is spontaneous. T: C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


