Question: 13. Which alcohol has a higher boiling point? a. (i) 2-methylpropan-2-ol or (i) butan-2-ol b. (i) hexan-1-ol or (i) 3,3-dimethylbutan-1-ol 14. Predict which member
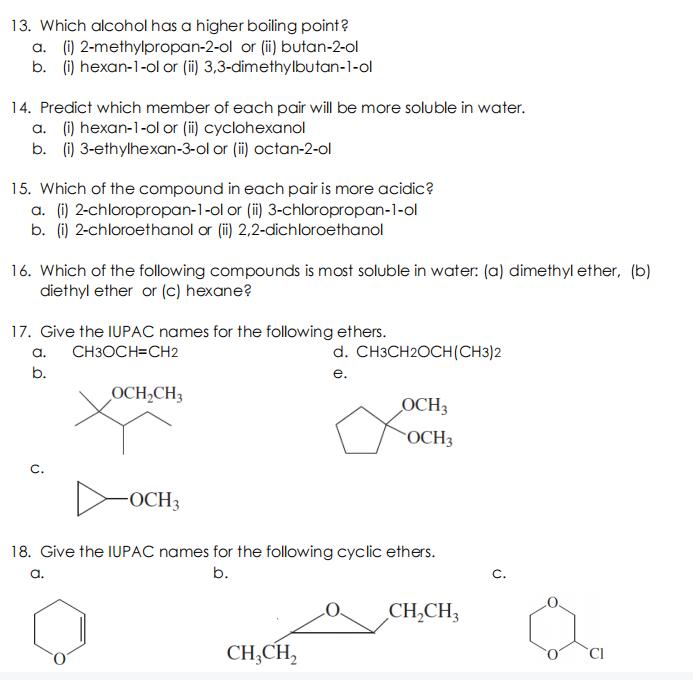
13. Which alcohol has a higher boiling point? a. (i) 2-methylpropan-2-ol or (i) butan-2-ol b. (i) hexan-1-ol or (i) 3,3-dimethylbutan-1-ol 14. Predict which member of each pair will be more soluble in water. a. (i) hexan-1-ol or (i) cyclohexanol b. (i) 3-ethylhexan-3-ol or (i) octan-2-ol 15. Which of the compound in each pair is more acidic? a. (i) 2-chloropropan-1-ol or (i) 3-chloropropan-1-ol b. (i) 2-chloroethanol or (i) 2,2-dichloroethanol 16. Which of the following compounds is most soluble in water: (a) dimethyl ether, (b) diethyl ether or (c) hexane? 17. Give the IUPAC names for the following ethers. a. CH3OCH=CH2 d. CH3CH2OCH(CH3)2 b. . OCH,CH3 OCH3 OCH3 C. OCH3 18. Give the IUPAC names for the following cyclic ethers. a. b. C. CH,CH3 CH,CH,
Step by Step Solution
3.50 Rating (160 Votes )
There are 3 Steps involved in it
w predict whic h member of each paiy coill be more Soluble in ... View full answer

Get step-by-step solutions from verified subject matter experts


