Question: 13-11 The equilibrium constant for the conjugate acid-base pair HIn+HOH,O + In is 8.00 x 105, From the additional information in the following table,
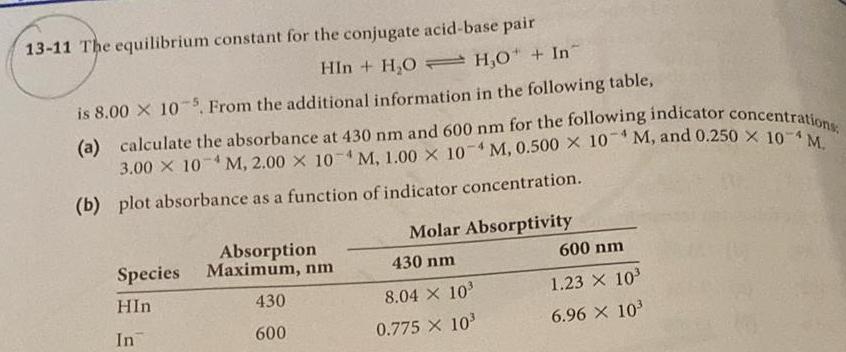
13-11 The equilibrium constant for the conjugate acid-base pair HIn+HOH,O + In is 8.00 x 105, From the additional information in the following table, (a) calculate the absorbance at 430 nm and 600 nm for the following indicator concentrations 3.00 X 10 M, 2.00 X 10M, 1.00 X 10 M, 0.500 X 10M, and 0.250 X 10M. 4 (b) plot absorbance as a function of indicator concentration. Species HIn Absorption Maximum, nm Molar Absorptivity 430 nm 600 nm 430 8.04 X 103 1.23 X 10 In 600 0.775 X 10 6.96 103
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


