Question: [14] 8. (a) For each compound below, how many signals would you expect the molecule to have in a 1HNMR and 13CNMR spectra? i.e. how
![[14] 8. (a) For each compound below, how many signals would](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8f278a1569_03266f8f278585e6.jpg)
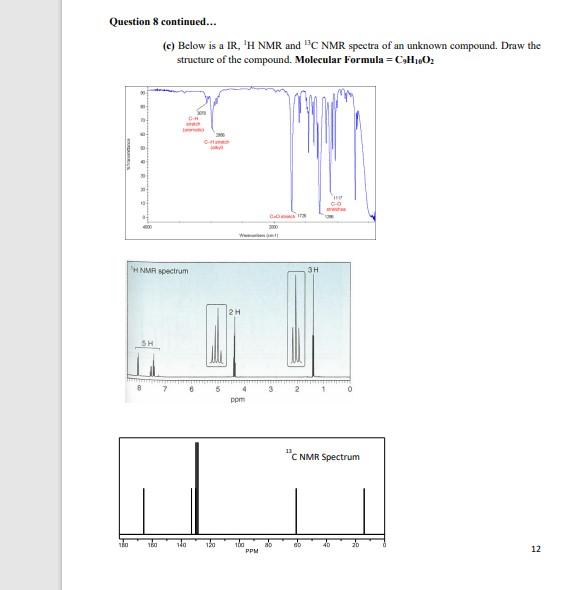
[14] 8. (a) For each compound below, how many signals would you expect the molecule to have in a 1HNMR and 13CNMR spectra? i.e. how many different types of protons and carbons are there? i) ii) iii) IH 13C (b) What splitting patterns would you expect from the protons at the indicated positions below? on 8 continued... (c) Below is a IR, 1H NMR and 13C NMR spectra of an unknown compound. Draw the structure of the compound. Molecular Formula =C9H16O2
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


