Question: Help!! Are these correct??? Please check over them. C 1. How many different 'H NMR signals are expected for this molecule? CH3C(O)CH2CH2CH3 A. 3 signals

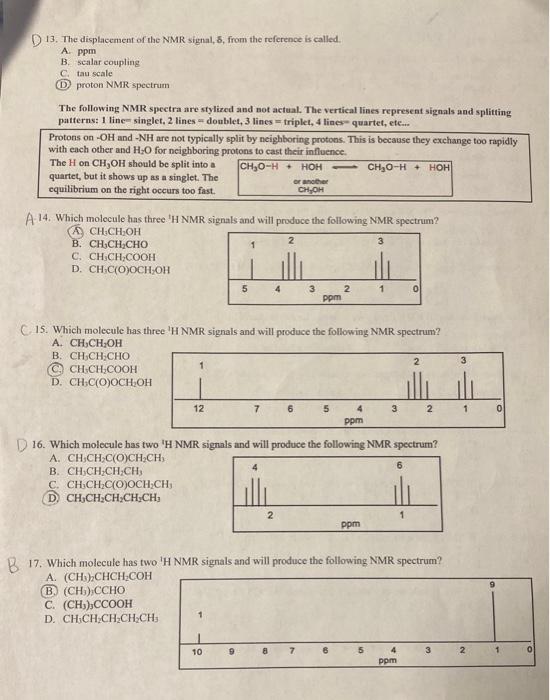
C 1. How many different 'H NMR signals are expected for this molecule? CH3C(O)CH2CH2CH3 A. 3 signals in a 2:2:3 ratio C.) 4 signals in a 3:2:2:3 ratio B. 3 signals in a 3:2:3 ratio D. 4 signals in a 3:3:2:3 ratio 2. How many different C NMR signals are expected for this molecule? CH3C(O)CH2CH2CH3 A. 2 Sis 4 B. 3 (D) 5 B. 3. How many different ' 1H NMR signals are expected for this molecule? (CH3)2CHOH A. 3 signals in a 1:1:3 ratio C. 4 signals in a 1:1:3:3 ratio (B.) 3 signals in a 1:1:6 ratio D. 4 signals in a 1:1:3:6 ratio A. 4. How many different 'H NMR signals are expected for this molecule? CH3C(O)OCH(CH3)/2 (C.) 3 signals in a 6:1:3 ratio C. 4 signals in a 3:3:1:3 ratio D. 3 signals in a 3:6:3 ratio D. 4 signals in a 3:1:6:3 ratio 5. How many different 13C NMR signals are expected for this molecule? CH3C(O)OCH(CH3)2 E. 1 C. 3 F. 2 (D.) 4 6. How many different 'H NMR signals are expected for this molecule? HOCH2CH(CHC3)2 A. 5 signals in a 3:3:2:1:1 ratio (C) 4 signals in a 6:1:2:1 ratio B. 3 signals in a 6:2:1 ratio D. 3 signals in a 3:6:1 ratio 7. How many different 'H NMR signals are expected for this molecule? CHCl3CHClCHCl2 (A.) 2 signals in a 2:1 ratio C. 3 signals in a 1:1:1 ratio B. 2 signals in a 1:1 ratio D. 2 signals in a 2:1:2 ratio 8. How many 1H NMR signals are expected for this compound? CH3CH2Cl A. 1 C. 3 (B) 2 D. 4 9. How many 1HNMR signals are expected for this compound? CH3CHClCH3 A. 1 C. 3 (B) 2 D. 4 10. How many 'H NMR signals are expected for this compound? CHClH=CH2 A. 1 C. 3 (B) 2 D. 4 11. How many 'H NMR signals are expected for this compound? CH3CHClCH2Cl A. 1 (C.) 3 B. 2 D. 4 12. How many 1H NMR signals are expected for this compound? (CH3)2C2CH2 A. 1 C. 3 (B) 2 D. 4 D 13. The displacement of the NMR signal, 8, from the reference is called. A. Ppm B. Scalar coupling C. tau scale (D) proton NMR spectrum The following NMR spectra are stylized and not actual. The vertical lines represent signals and splitting patterns: 1 line- singlet, 2 lines = doublet, 3 lines = triplet, 4 liaes- quartet, ete.. Protons on - OH and -NH are not typically split by neighboring protons. This is because they exchange too rapidly with each other and H2O for neighboring protons to cast their influence. The H on CH3OH should be split into a quartet, but it shows up as a singlet. The equilibrium on the right occurs too fast
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


