Question: (14%) Problem 5: A mass m = 4 .75 kg of lava at TH = 113335'3 C emerges from a volcano deep in the ocean.

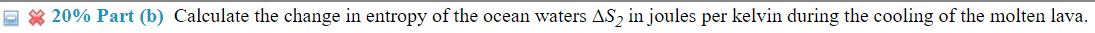
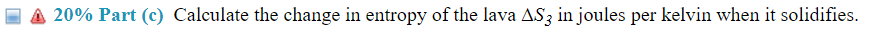
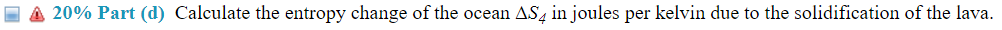

(14%) Problem 5: A mass m = 4 .75 kg of lava at TH = 113335'3 C emerges from a volcano deep in the ocean. At this depth, the ocean water is at a temperature of T C = 2. 5 C, and the lava cools and solidies. The specic heat capacity of the lava for this process is c = 840 J/kg-C and the latent heat of fusion of the lava is Lf= 4.0 X 105 Ja'kg. Assume that the temperature of the ocean does not change in this process. g 33 20% Part (b) Calculate the change in entropy of the ocean waters A82 injoules per kelvin during the cooling of the molten lava. E 3 20% Part (1:) Calculate the change in entropy of the lava 5.33 injoules per kelvin when it solidies. E 3 20% Part ((1) Calculate the entropy change of the ocean A34 injoules per kelvin due to the solidication of the lava. \f
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


