Question: 14. The specific enthalpy of a certain liquid binary phase of A and B is given by h=KAx+Ke(1-x)+bx(1-x2), where x is the mol fraction of
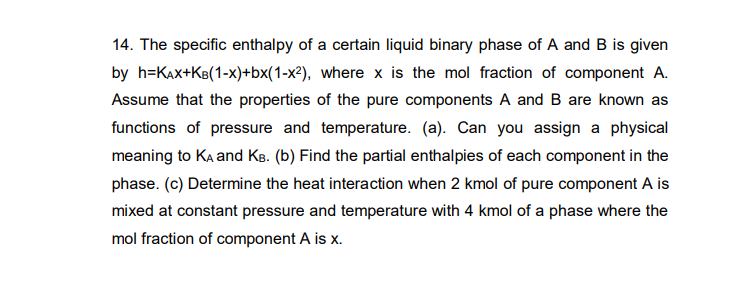
14. The specific enthalpy of a certain liquid binary phase of A and B is given by h=KAx+Ke(1-x)+bx(1-x2), where x is the mol fraction of component A. Assume that the properties of the pure components A and B are known as functions of pressure and temperature. (a). Can you assign a physical meaning to Ka and KB. (b) Find the partial enthalpies of each component in the phase. (c) Determine the heat interaction when 2 kmol of pure component A is mixed at constant pressure and temperature with 4 kmol of a phase where the mol fraction of component A is X. 14. The specific enthalpy of a certain liquid binary phase of A and B is given by h=KAx+Ke(1-x)+bx(1-x2), where x is the mol fraction of component A. Assume that the properties of the pure components A and B are known as functions of pressure and temperature. (a). Can you assign a physical meaning to Ka and KB. (b) Find the partial enthalpies of each component in the phase. (c) Determine the heat interaction when 2 kmol of pure component A is mixed at constant pressure and temperature with 4 kmol of a phase where the mol fraction of component A is X
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


