Question: 14. Using pKa values from the table, determine the direction of equilibrium for each of the following two proton transfer reactions: (a) (b) 15. Which
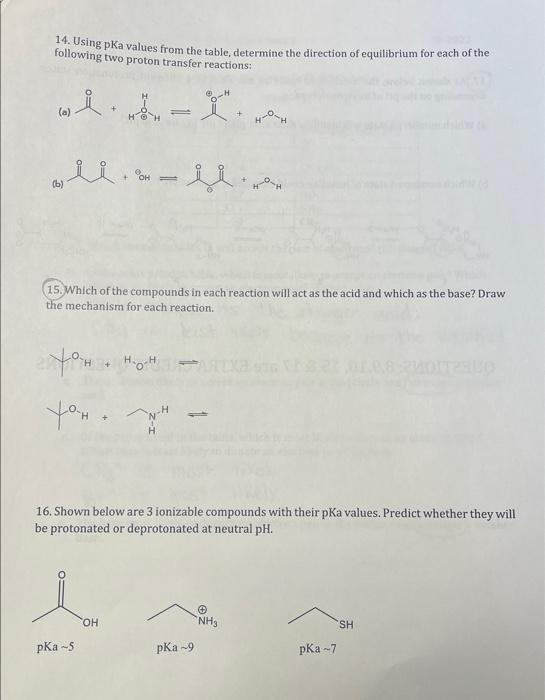
14. Using pKa values from the table, determine the direction of equilibrium for each of the following two proton transfer reactions: (a) (b) 15. Which of the compounds in each reaction will act as the acid and which as the base? Draw the mechanism for each reaction. 16. Shown below are 3 ionizable compounds with their pKa values. Predict whether they will be protonated or deprotonated at neutral pH. pKa5 pKa 9 pKa7
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


