Question: 1.49gH2 is allowed to react with 10.4gNN2, producing 1.49gNH3. The Haber-Bosch process is a very important industrial process. In the Haber-Bosch process, hydrogen gas reacts
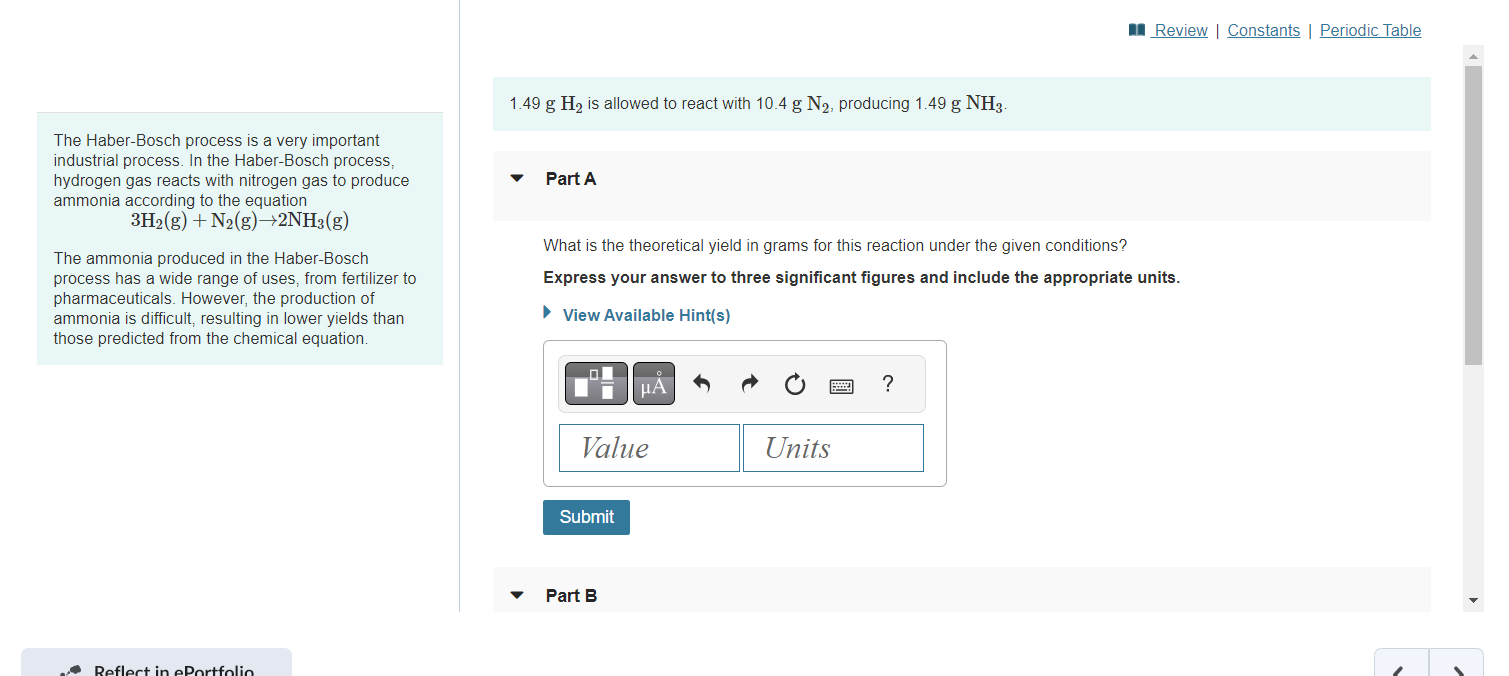
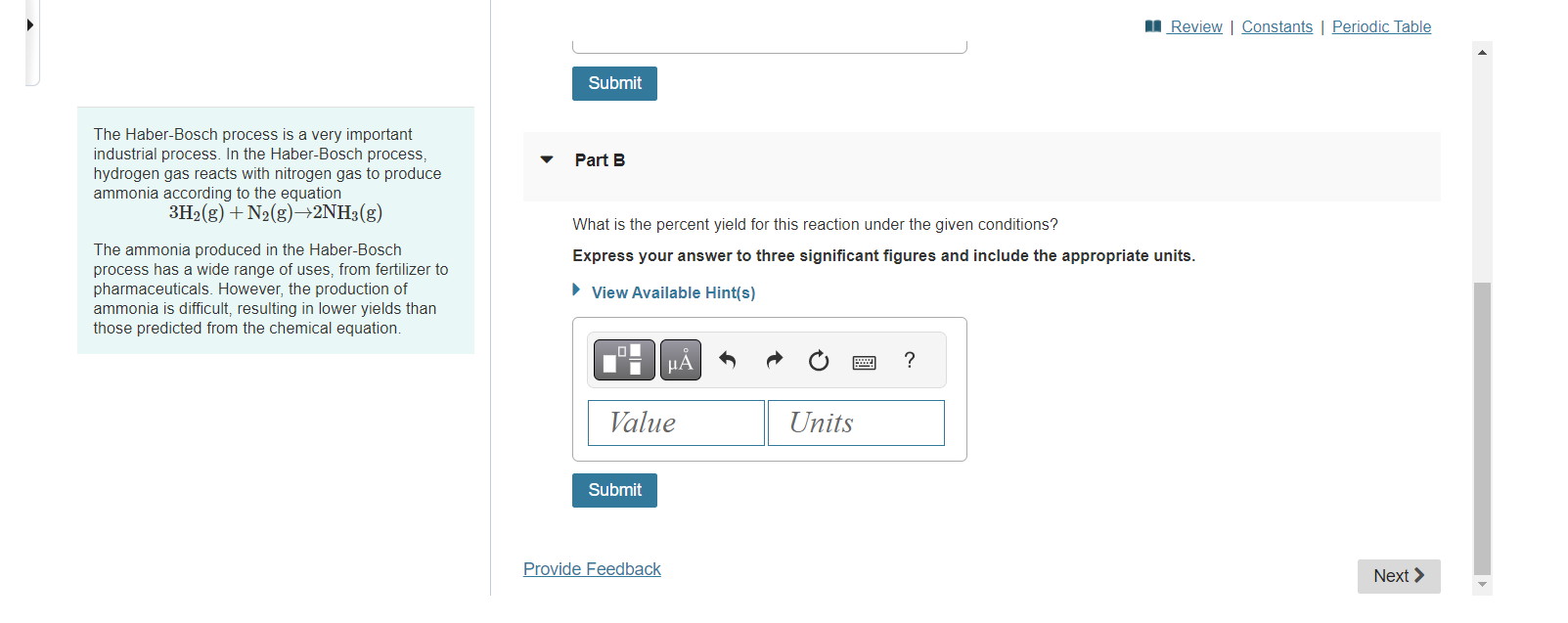
1.49gH2 is allowed to react with 10.4gNN2, producing 1.49gNH3. The Haber-Bosch process is a very important industrial process. In the Haber-Bosch process, hydrogen gas reacts with nitrogen gas to produce Part A ammonia according to the equation 3H2(g)+N2(g)2NH3(g) The ammonia produced in the Haber-Bosch What is the theoretical yield in grams for this reaction under the given conditions? process has a wide range of uses, from fertilizer to Express your answer to three significant figures and include the appropriate units. pharmaceuticals. However, the production of ammonia is difficult, resulting in lower yields than those predicted from the chemical equation. The Haber-Bosch process is a very important industrial process. In the Haber-Bosch process, hydrogen gas reacts with nitrogen gas to produce ammonia according to the equation 3H2(g)+N2(g)2NH3(g) What is the percent yield for this reaction under the given conditions? The ammonia produced in the Haber-Bosch process has a wide range of uses, from fertilizer to Express your answer to three significant figures and include the appropriate units. pharmaceuticals. However, the production of ammonia is difficult, resulting in lower yields than those predicted from the chemical equation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


