Question: 1.90gH2 is allowed to react with 9.75gN2, producing 2.91gNH3. The Haber-Bosch process is a very important industrial orocess. In the Haber-Bosch process, hydrogen gas reacts
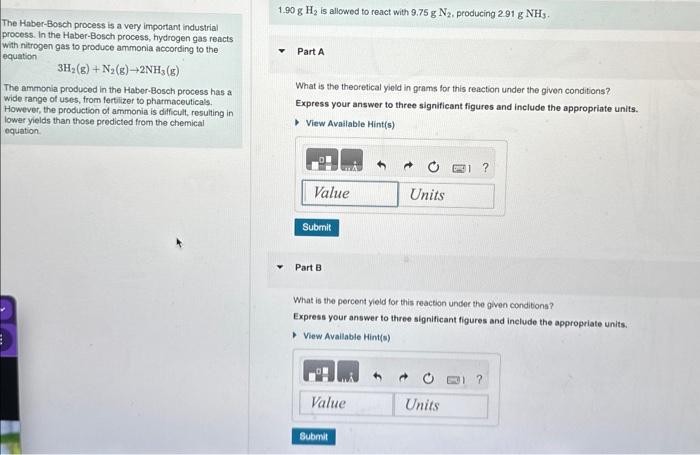
1.90gH2 is allowed to react with 9.75gN2, producing 2.91gNH3. The Haber-Bosch process is a very important industrial orocess. In the Haber-Bosch process, hydrogen gas reacts with nitrogen gas to produce ammonia according to the gquation 3H2(g)+N2(g)2NH3(g) The ammonia produced in the Haber-Bosch process has a wide range of uses, from fertilizer to pharmaceuticals. However, the production of ammonia is difficult, resulting in lower yields than those predicted from the chemical equation. Part A What is the theoretical yield in grams for this reaction under the given conditions? Express your answer to three significant figures and include the appropriate units. Part B What is the percent yleld for this reaction under the given conditions? Express your answer to three significant figures and include the appropriate units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


