Question: 1.5 1 O/ HO C 0.5 Potential, E/V pH = 4 Natural waters 6 = Hd D -0.5 E H/H, F -1 0 4 8
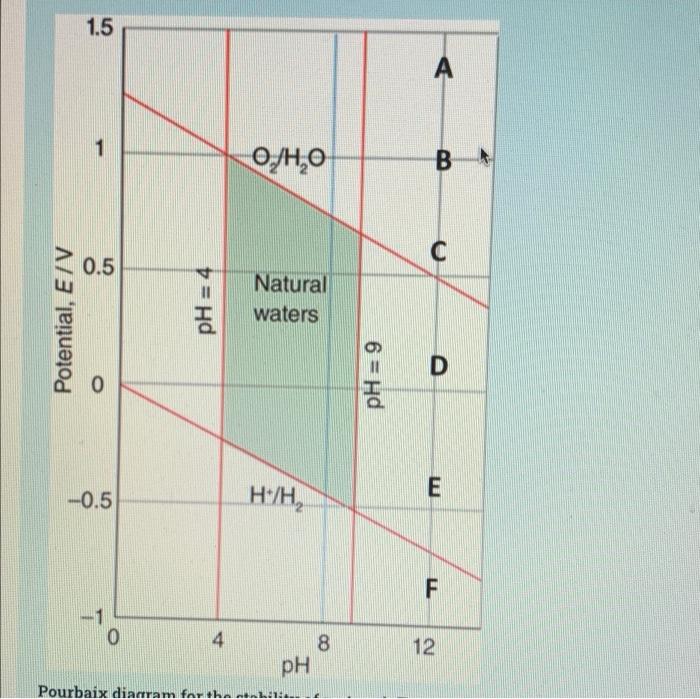

1.5 1 O/ HO C 0.5 Potential, E/V pH = 4 Natural waters 6 = Hd D -0.5 E H/H, F -1 0 4 8 8 pH 12 Pourbaix diagram for the 0 12 8 PH Pourbaix diagram for the stability of water, A-F represent species position at the diagram based on their reduction potential at pH=12. Based on Pourbaix diagram for the stability field of water answer the following questions: 1. For the natural water, the reduction potential (V) within the stability field of water at pH=9 is approximately between e 2- For the species A-F, the one that can oxidize water at any pH is e 3. For the species A-F, the one that can oxidize water at pH=12 or more is 4 4- For the species A-F, the one that is stable in water at all pH is 5- For the species A-F, the one that can reduce water at any pH is
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


