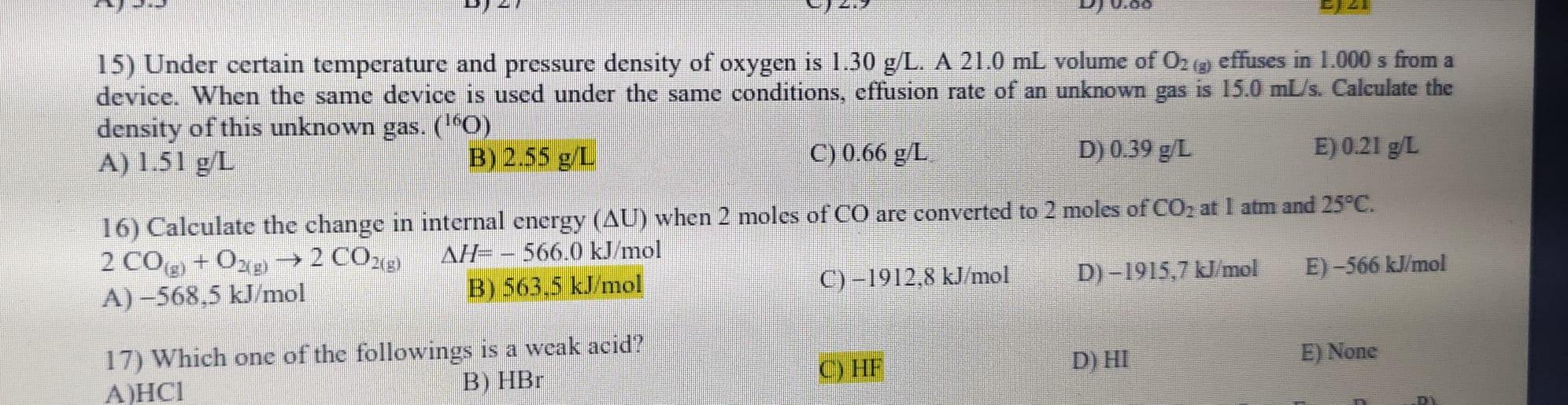
Question: 15 and 16 ?? 15) Under certain temperature and pressure density of oxygen is 1.30 g/L. A 21.0 mL volume of Oz (g) effuses in
 15 and 16 ??
15 and 16 ??
15) Under certain temperature and pressure density of oxygen is 1.30 g/L. A 21.0 mL volume of Oz (g) effuses in 1.000 s from a device. When the same device is used under the same conditions, effusion rate of an unknown gas is 15.0 mL/s. Calculate the density of this unknown gas. (10) A) 1.51 g/L B) 2.55 g/L C) 0.66 g/L D) 0.39 g/L E) 0.21 g/L 16) Calculate the change in internal energy (AU) when 2 moles of CO are converted to 2 moles of CO2 at 1 atm and 25C. 2 C0g) + 029 2 CO2(g) AH=- 566.0 kJ/mol A)-568,5 kJ/mol B) 563.5 kJ/mol C) -1912.8 kJ/mol D) 1915,7 kJ/mol E) -566 kJ/mol D) HI E) None 17) Which one of the followings is a weak acid? ) B) HBr C) HF
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


