Question: (15 pts) A reaction has 3 elementary steps: (1) H_2 (g)+ O_2 (g) (k_1 ) H_2 O_2 (g) (slow) (2) H_2 O_2 (g) (k_2 )
(15 pts) A reaction has 3 elementary steps: (1) H_2 (g)+ O_2 (g) (k_1 ) H_2 O_2 (g) (slow) (2) H_2 O_2 (g) (k_2 ) H_2 O(g)+ O(g) (fast) (3) O(g)+ H_2 (g) (k_3 ) H_2 O(g) (fast) Which step is the rate-limiting step? What is the overall reaction? What are the intermediates? Derive an expression for water formation.
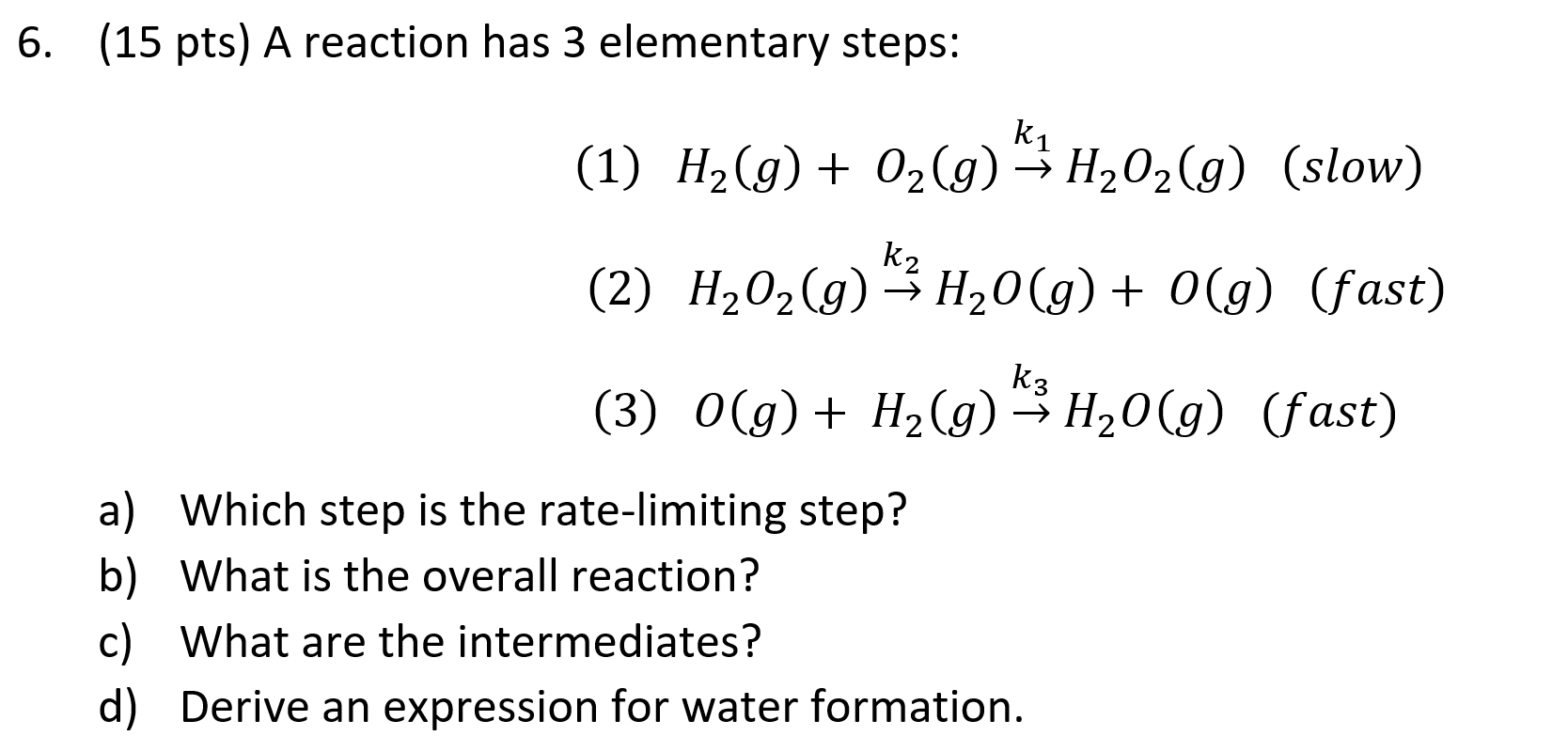
6. (15 pts) A reaction has 3 elementary steps: (1) H2(g)+O2(g)k1H2O2(g) (slow) (2) H2O2(g)k2H2O(g)+O(g) (fast) (3) O(g)+H2(g)k3H2O(g) (fast) a) Which step is the rate-limiting step? b) What is the overall reaction? c) What are the intermediates? d) Derive an expression for water formation
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


