Question: 16. Consider a gas mixture whose apparent moleular weight is 33, initially at 3 bar and 300K, and occupying a vohme of 0.1 m.
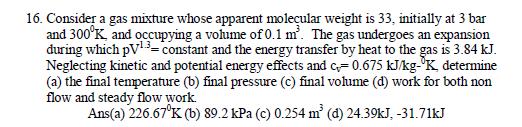
16. Consider a gas mixture whose apparent moleular weight is 33, initially at 3 bar and 300K, and occupying a vohme of 0.1 m. The gas undergoes an expansion during which pV13-constant and the energy transfer by heat to the gas is 3.84 kJ. Neglecting kinetic and potential energy effects and c0.675 kJkgK determine (a) the final temperature (b) final pressure (c) final volume (d) work for both non flow and steady flow work. Ans(a) 226.67K (b) 89.2 kPa (c) 0.254 m (d) 24.39kJ, -31.71kJ
Step by Step Solution
3.35 Rating (155 Votes )
There are 3 Steps involved in it
To solve this problem well follow these steps Given Apparent molecular weight MW 33 Initial pressure ... View full answer

Get step-by-step solutions from verified subject matter experts


