Question: Homework set # 2 due by Sep 7 A gas is compressed from V 1 = 0 . 3 m 3 , p 1 =
Homework set # due by Sep
A gas is compressed from bar to bar. Pressure and volume are related linearly
during the process. For the gas, find the work, in kJ
A closed system consisting of lbmol of air undergoes a polytropic process from to a
final state where Determine the amount of energy transfer by work, in Btu for the process.
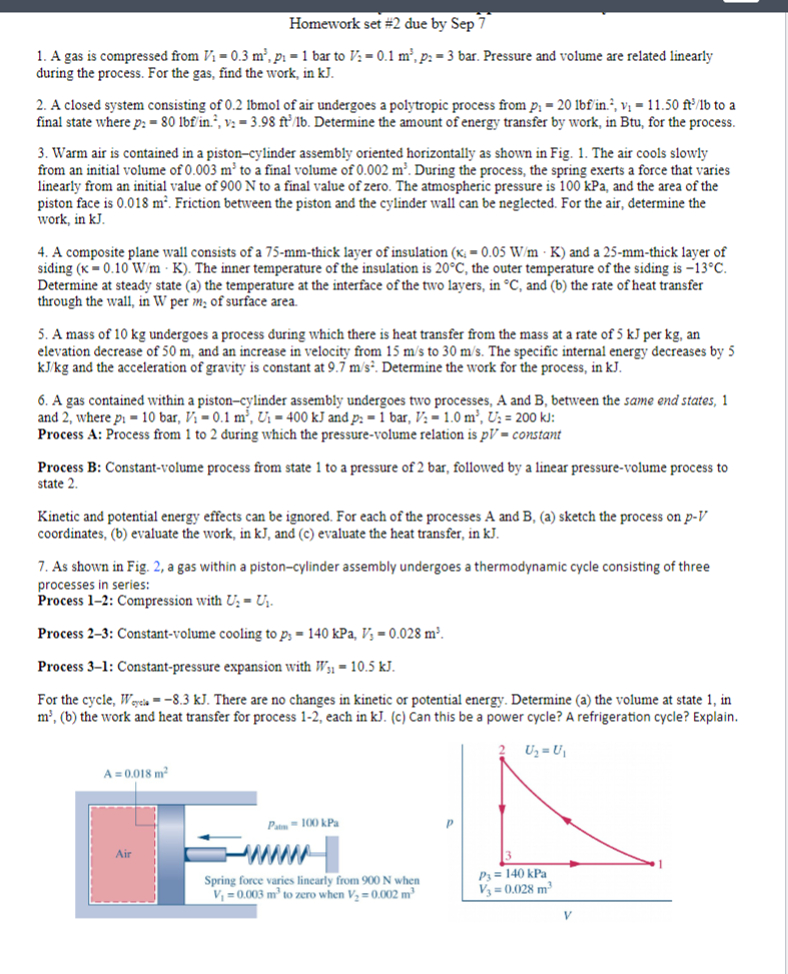
Warm air is contained in a pistoncylinder assembly oriented horizontally as shown in Fig. The air cools slowly
from an initial volume of to a final volume of During the process, the spring exerts a force that varies
linearly from an initial value of N to a final value of zero. The atmospheric pressure is kPa and the area of the
piston face is Friction between the piston and the cylinder wall can be neglected. For the air, determine the
work, in kJ
A composite plane wall consists of a mmthick layer of insulation and a mmthick layer of
siding The inner temperature of the insulation is the outer temperature of the siding is
Determine at steady state a the temperature at the interface of the two layers, in and b the rate of heat transfer
through the wall, in W per of surface area.
A mass of kg undergoes a process during which there is heat transfer from the mass at a rate of kJ per kg an
elevation decrease of m and an increase in velocity from to The specific internal energy decreases by
and the acceleration of gravity is constant at Determine the work for the process, in kJ
A gas contained within a pistoncylinder assembly undergoes two processes, A and B between the same end states,
and where bar, and bar, :
Process A: Process from to during which the pressurevolume relation is constant
Process B: Constantvolume process from state to a pressure of bar, followed by a linear pressurevolume process to
state
Kinetic and potential energy effects can be ignored. For each of the processes A and B a sketch the process on
coordinates, b evaluate the work, in kJ and c evaluate the heat transfer, in kJ
As shown in Fig. a gas within a pistoncylinder assembly undergoes a thermodynamic cycle consisting of three
processes in series:
Process : Compression with
Process : Constantvolume cooling to kPa,
Process : Constantpressure expansion with
For the cycle, There are no changes in kinetic or potential energy. Determine a the volume at state in
b the work and heat transfer for process each in kJ c Can this be a power cycle? A refrigeration cycle? Explain.
Spring force varies linearly from N when
to zero when
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


