Question: 17.2.1 Question 5 Consider the final unit operation (an absorption tower) for the production of an aqueous liquid ammonia product. Stream S5 is a reaction
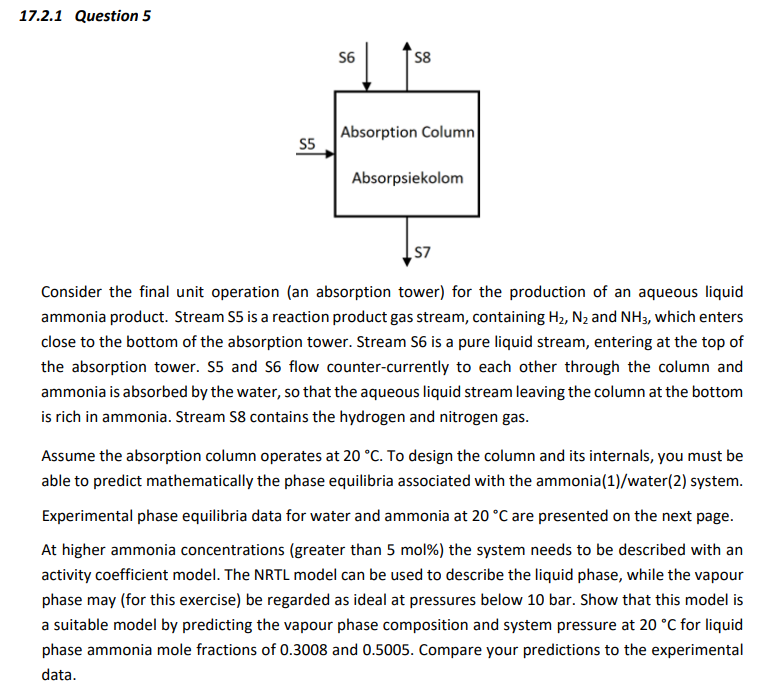
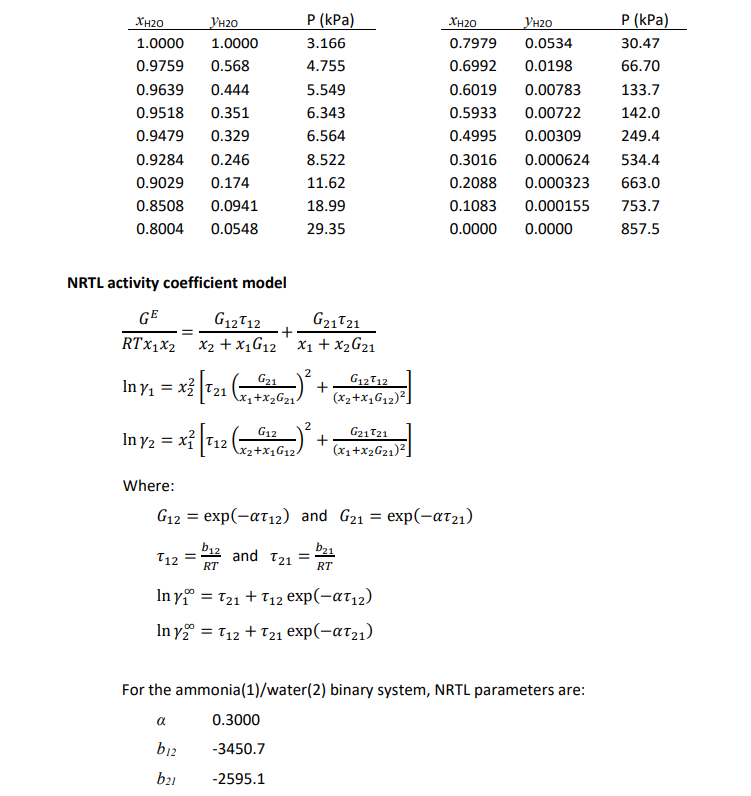
17.2.1 Question 5 Consider the final unit operation (an absorption tower) for the production of an aqueous liquid ammonia product. Stream S5 is a reaction product gas stream, containing H2,N2 and NH3, which enters close to the bottom of the absorption tower. Stream S6 is a pure liquid stream, entering at the top of the absorption tower. S5 and S6 flow counter-currently to each other through the column and ammonia is absorbed by the water, so that the aqueous liquid stream leaving the column at the bottom is rich in ammonia. Stream S8 contains the hydrogen and nitrogen gas. Assume the absorption column operates at 20C. To design the column and its internals, you must be able to predict mathematically the phase equilibria associated with the ammonia(1)/water(2) system. Experimental phase equilibria data for water and ammonia at 20C are presented on the next page. At higher ammonia concentrations (greater than 5 mol\%) the system needs to be described with an activity coefficient model. The NRTL model can be used to describe the liquid phase, while the vapour phase may (for this exercise) be regarded as ideal at pressures below 10 bar. Show that this model is a suitable model by predicting the vapour phase composition and system pressure at 20C for liquid phase ammonia mole fractions of 0.3008 and 0.5005 . Compare your predictions to the experimental data. NRTL activity coefficient model RTx1x2GE=x2+x1G12G1212+x1+x2G21G2121ln1=x22[21(x1+x2G21G21)2+(x2+x1G12)2G1212]ln2=x12[12(x2+x1G12G12)2+(x1+x2G21)2G2121] Where: G12=exp(12)andG21=exp(21)12=RTb12and21=RTb21ln1=21+12exp(12)ln2=12+21exp(21) For the ammonia(1)/water(2) binary system, NRTL parameters are: b12b210.30003450.72595.1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


