Question: 1.How much did the freezing point go down when the solute was added to the lauric acid? In other words, what was the change in
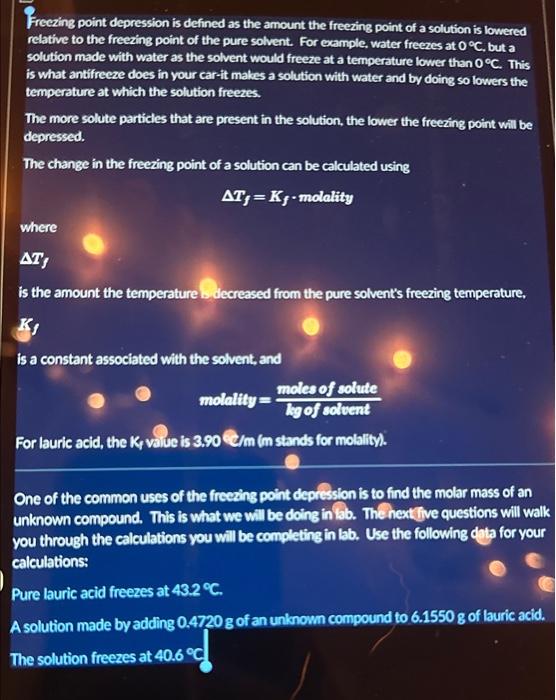
Freezing point depression is defined as the amount the freezing point of a solution is lowered relative to the freezing point of the pure solvent. For example, water freezes at 0C, but a solution made with water as the solvent would freeze at a temperature lower than 0C. This is what antifreeze does in your car-it makes a solution with water and by doing so lowers the temperature at which the solution freezes. The more solute particles that are present in the solution, the lower the freezing point will be depressed. The change in the freezing point of a solution can be calculated using Tf=Kf-molality where If is the amount the temperature Lidecreased from the pure solvent's freezing temperature, is a constant associated with the solvent, and molality=kgofsolventmolesofsolute For lauric acid, the K, value is 3,90c/m (m stands for molality). One of the common uses of the freezing point depression is to find the molar mass of an unknown compound. This is what we will be doing in ib. The next five questions will wall you through the calculations you will be completing in lab. Use the following data for your calculations: Pure lauric acid freezes at 43.2C. A solution made by adding 0.4720g of an unknown compound to 6.1550g of lauric acid. The solution freezes at 40.6C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


