Question: 2 # 1. The irreversible reaction (AB) was studied in a batch reactor (CAo=2.0 mol/Li ). Figure 1 (next page) shows the kinetic data obtained.
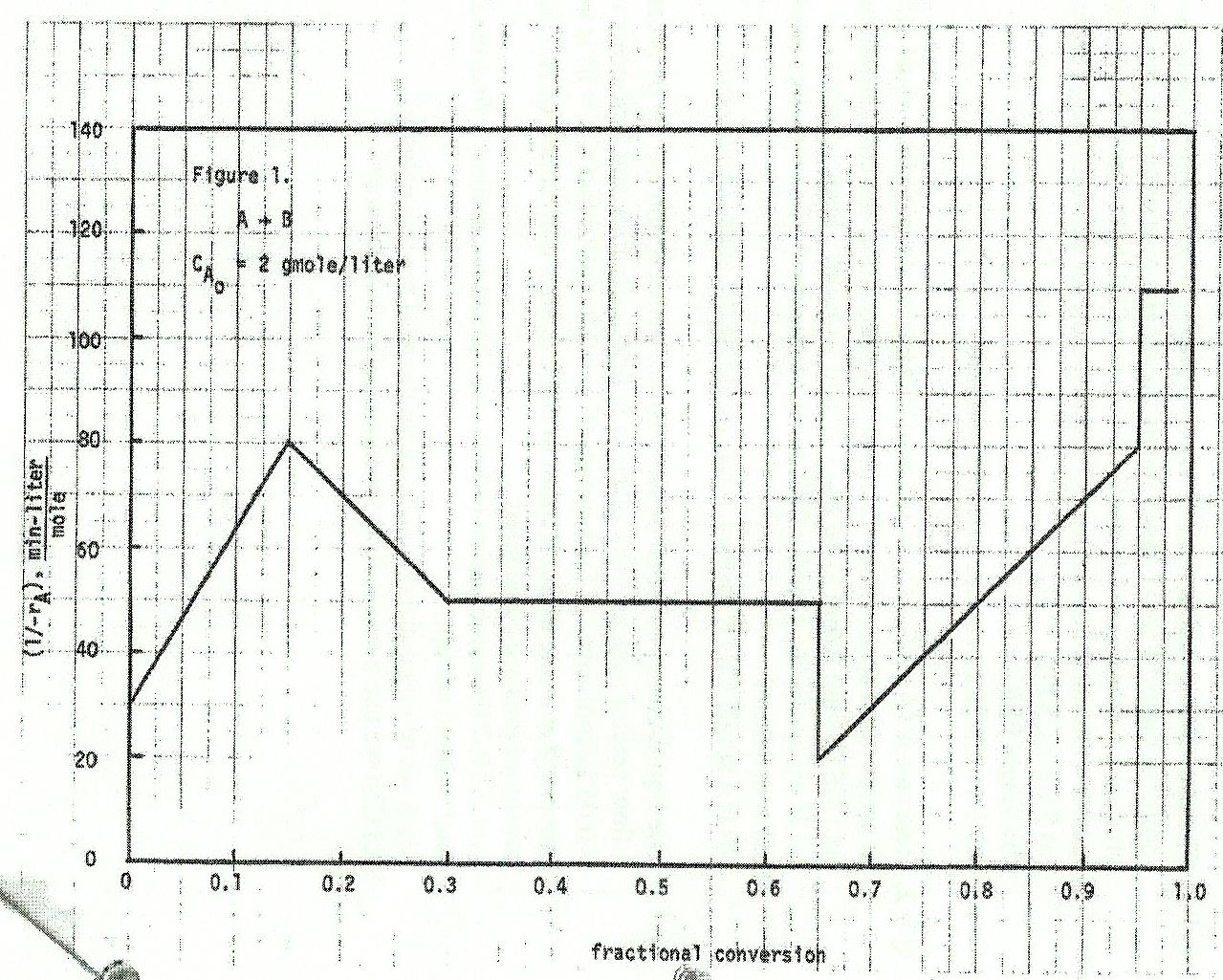
2 \# 1. The irreversible reaction (AB) was studied in a batch reactor (CAo=2.0 mol/Li ). Figure 1 (next page) shows the kinetic data obtained. We wish to process a feed ( CAo=1.0mol/Li ) at a rate of 10,000Li/hr at 40C. Find the reactor volume(s) needed for 90% conversion for the following configurations: (a) Single CSTR (b) Single PFR (c) Two CSTRs in series of equal volumes
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


