Question: 2. [1-component phase behavior] ( 30 points) Table 1 presents vapor pressures and molar volumes of the liquid and vapor phases for n-butane. Figure 1
![2. [1-component phase behavior] ( 30 points) Table 1 presents vapor](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66e41c99cefb0_56166e41c996d0bd.jpg)
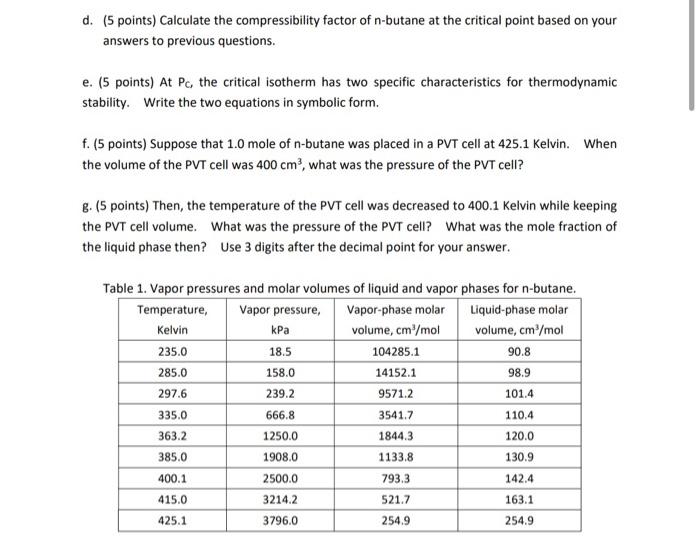
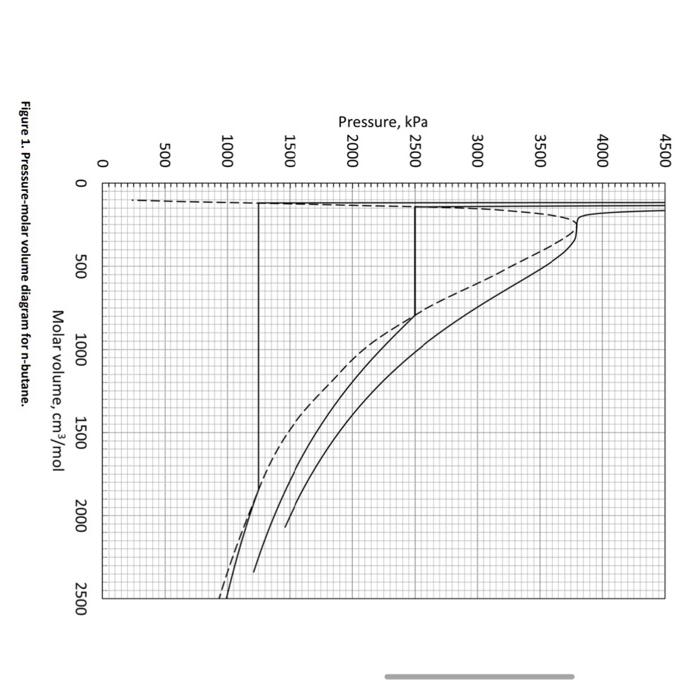
2. [1-component phase behavior] ( 30 points) Table 1 presents vapor pressures and molar volumes of the liquid and vapor phases for n-butane. Figure 1 shows three isotherms for n butane. The universal gas constant R is 8314.5(cm3kPaK1mol1). a. (3 points) Find the critical temperature ( Tc), critical pressure ( Pc), and critical molar volume (VC) of n-butane in kPa, Kelvin, and cm3/mol, respectively. b. ( 2 points) Determine the acentric factor of n-butane based on Table 1. =1.0log10[Pvap(Tr=0.7)/Pc)] c. (5 points) Based on Table 1, find the temperature for each of the three isotherms shown in the figure. d. (5 points) Calculate the compressibility factor of n-butane at the critical point based on your answers to previous questions. e. (5 points) At Pc, the critical isotherm has two specific characteristics for thermodynamic stability. Write the two equations in symbolic form. f. (5 points) Suppose that 1.0 mole of n-butane was placed in a PVT cell at 425.1 Kelvin. When the volume of the PVT cell was 400cm3, what was the pressure of the PVT cell? g. (5 points) Then, the temperature of the PVT cell was decreased to 400.1 Kelvin while keeping the PVT cell volume. What was the pressure of the PVT cell? What was the mole fraction of the liquid phase then? Use 3 digits after the decimal point for your answer. Molar volume, cm3/mol Figure 1. Pressure-molar volume diagram for n-butane
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


