Question: 2. (20 points) (a) Does the entropy increase, decrease or not change in the following processes: N2+3H22NH3 (b) G0 necessary for the synthesis of ATP
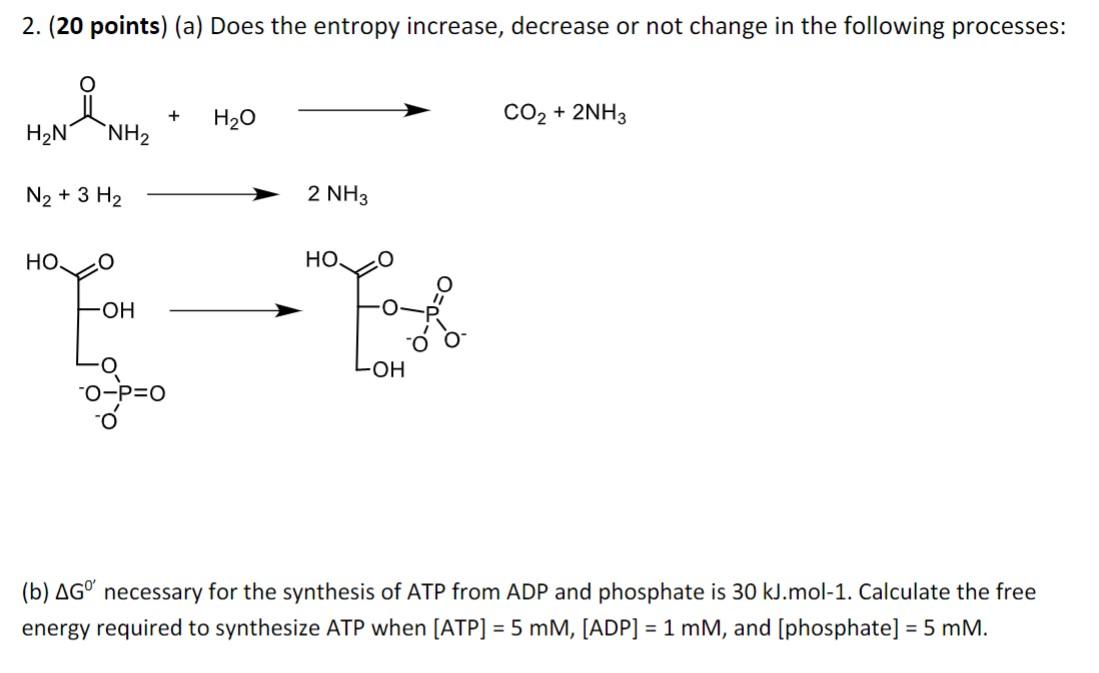
2. (20 points) (a) Does the entropy increase, decrease or not change in the following processes: N2+3H22NH3 (b) G0 necessary for the synthesis of ATP from ADP and phosphate is 30kJ.mol1. Calculate the free energy required to synthesize ATP when [ATP]=5mM,[ADP]=1mM, and [phosphate =5mM
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


