Question: 60. When aqueous NH3 is first added to a solution containing Ni2+, a precipitate forms, but when an excess of aqueous NH3 is added, the
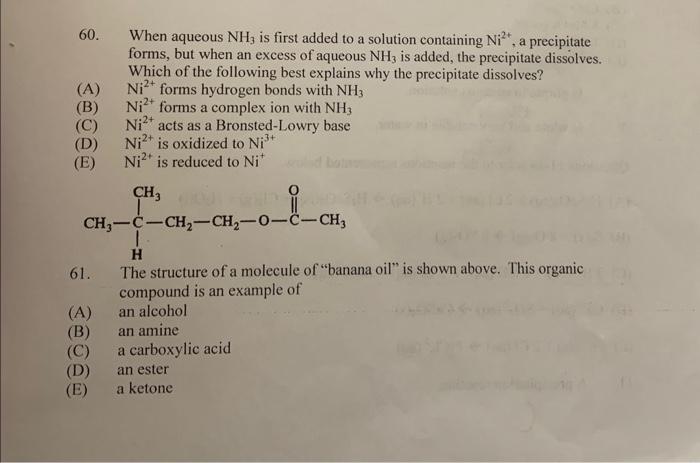
60. When aqueous NH3 is first added to a solution containing Ni2+, a precipitate forms, but when an excess of aqueous NH3 is added, the precipitate dissolves. Which of the following best explains why the precipitate dissolves? (A) Ni2+ forms hydrogen bonds with NH3 (B) Ni2+ forms a complex ion with NH3 (C) Ni2+ acts as a Bronsted-Lowry base (D) Ni2+ is oxidized to Ni3+ (E) Ni2+ is reduced to Ni+ 61. The structure of a molecule of "banana oil" is shown above. This organic compound is an example of (A) an alcohol (B) an amine (C) a carboxylic acid (D) an ester (E) a ketone
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


