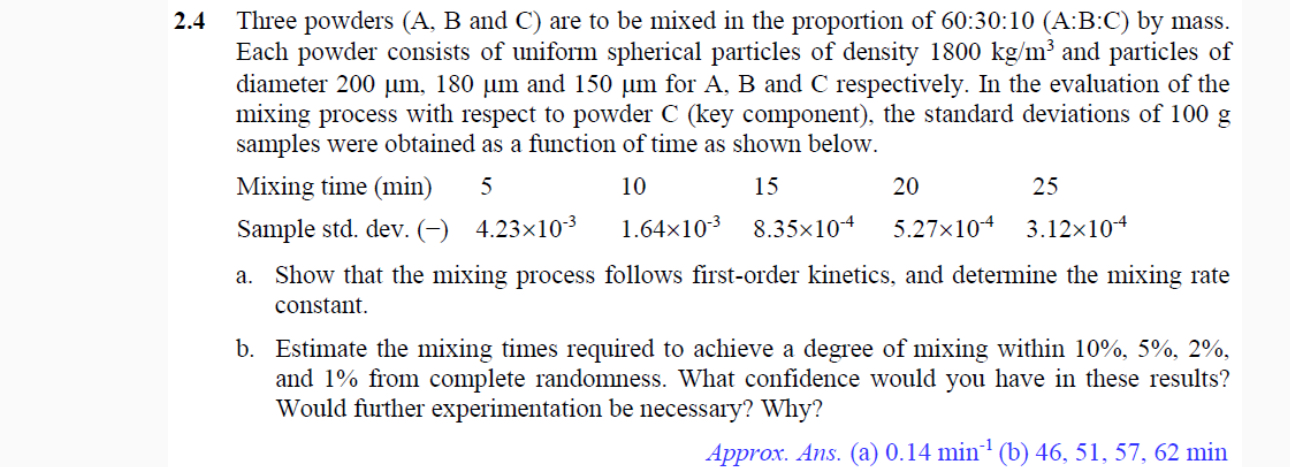
Question: 2 . 4 Three powders ( A , B and C ) are to be mixed in the proportion of 6 0 : 3 0
Three powders A B and C are to be mixed in the proportion of :::: by mass.
Each powder consists of uniform spherical particles of density and particles of
diameter and for A B and C respectively. In the evaluation of the
mixing process with respect to powder key component the standard deviations of
samples were obtained as a function of time as shown below.
a Show that the mixing process follows firstorder kinetics, and determine the mixing rate
constant.
b Estimate the mixing times required to achieve a degree of mixing within
and from complete randomness. What confidence would you have in these results?
Would further experimentation be necessary? Why?
Approx. Ans. abmin
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


