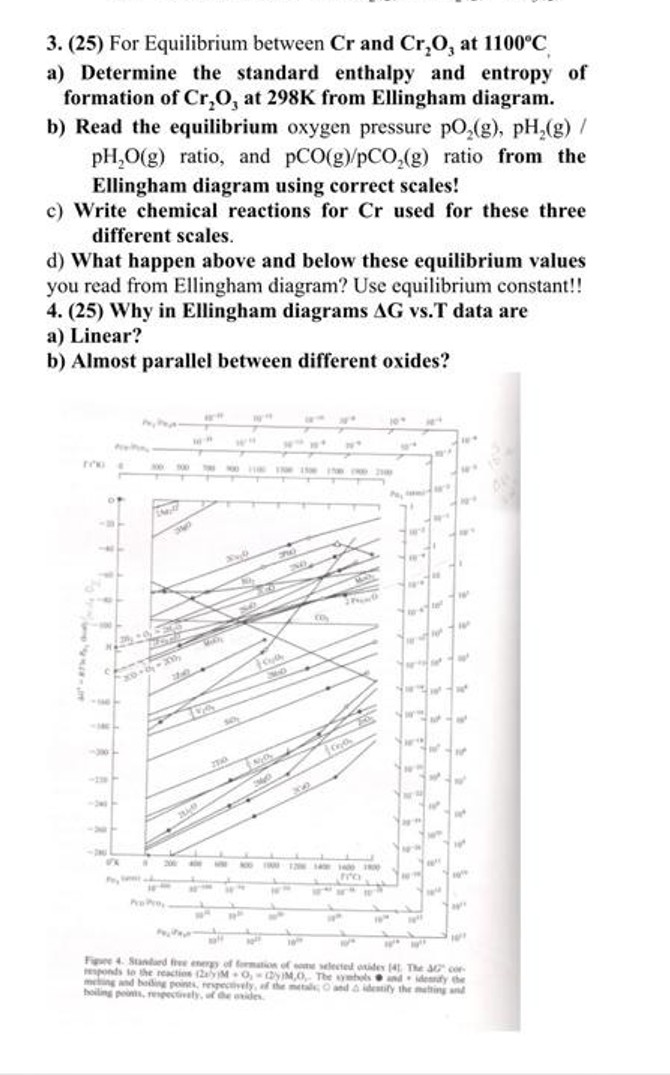
Question: ( 2 5 ) For Equilibrium between C r and C r 2 O 3 at 1 1 0 0 C a ) Determine the
For Equilibrium between and at
a Determine the standard enthalpy and entropy of
formation of at from Ellingham diagram.
b Read the equilibrium oxygen pressure
ratio, and pCO ratio from the
Ellingham diagram using correct scales!
c Write chemical reactions for used for these three
different scales.
d What happen above and below these equilibrium values
you read from Ellingham diagram? Use equilibrium constant!!
Why in Ellingham diagrams vsT data are
a Linear?
b Almost parallel between different oxides?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


