Question: 2. (8 points) Shown here is the phase diagram for compound XY2 molecule. The triple point of XY2 is 28.11C at 0.75 atm and the
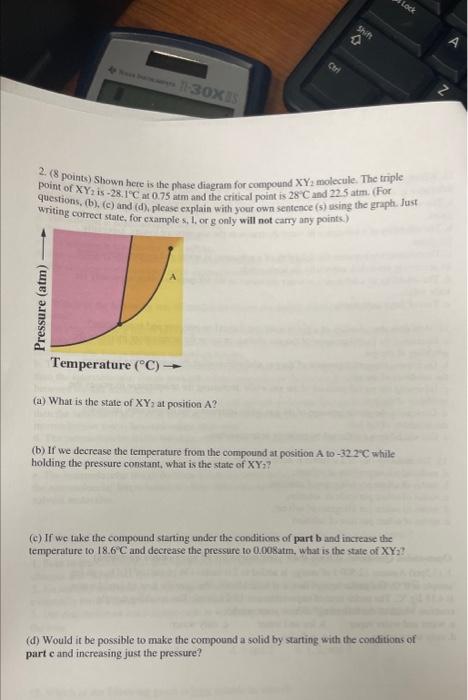
2. (8 points) Shown here is the phase diagram for compound XY2 molecule. The triple point of XY2 is 28.11C at 0.75 atm and the eritical point is 28C and 22.5 atm. (For questions, (b), (c) and (d), please explain with your own sentence (s) using the graph. Just writing correct state, for cxamole s. 1. or g only will not carry any points). (a) What is the state of XY2 at position A ? (b) If we decrease the temperature from the compound at position A to 322C while holding the pressure constant, what is the state of XY2 ? (c) If we take the compound starting under the conditions of part b and increase the temperature to 18.6C and decrease the pressure to 0.008atm, what is the state of XY ? (d) Would it be possible to make the compound a solid by starting with the conditions of part c and inereasing just the pressure
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


