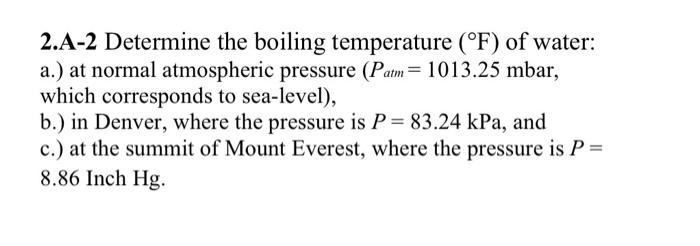
Question: 2 . A - 2 Determine the boiling temperature ( F ) of water: a . ) at normal atmospheric pressure ( Patm = 1
A Determine the boiling temperature F of water: a at normal atmospheric pressure Patmmbar, which corresponds to sealevel b in Denver, where the pressure is PkPa, and c at the summit of Mount Everest, where the pressure is P Inch Hg
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


