Question: 2. A 5mL container with a hole having a radius of 5106m is filled with hydrogen. This container is placed in an evacuated chamber at
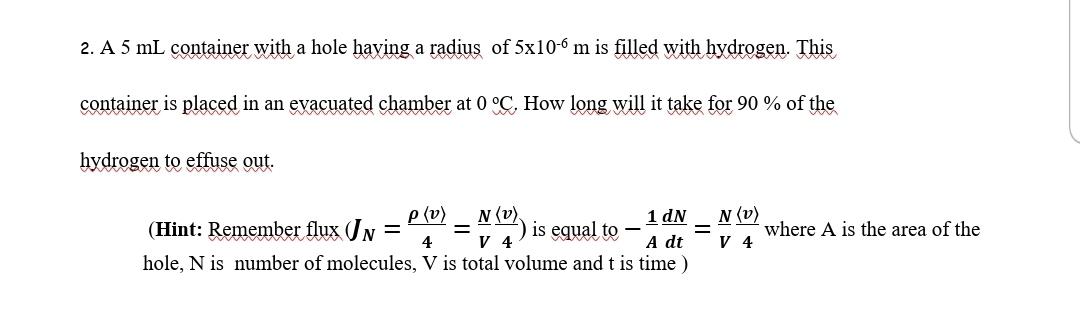
2. A 5mL container with a hole having a radius of 5106m is filled with hydrogen. This container is placed in an evacuated chamber at 0C. How long will it take for 90% of the hydrogen to effuse out. (Hint: Remember flux (JN=4v=VN4v) is equal to A1dtdN=VN4v where A is the area of the hole, N is number of molecules, V is total volume and t is time )
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


