Question: please help with tate constant. i have this equation but dont know what to use as values to find k. trying to find the rate
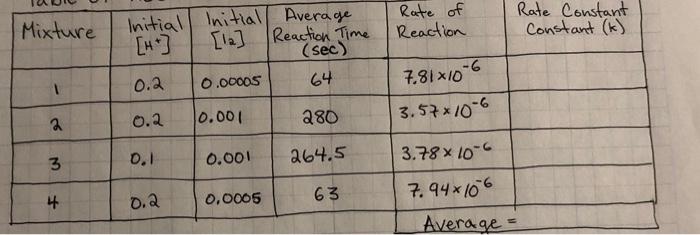

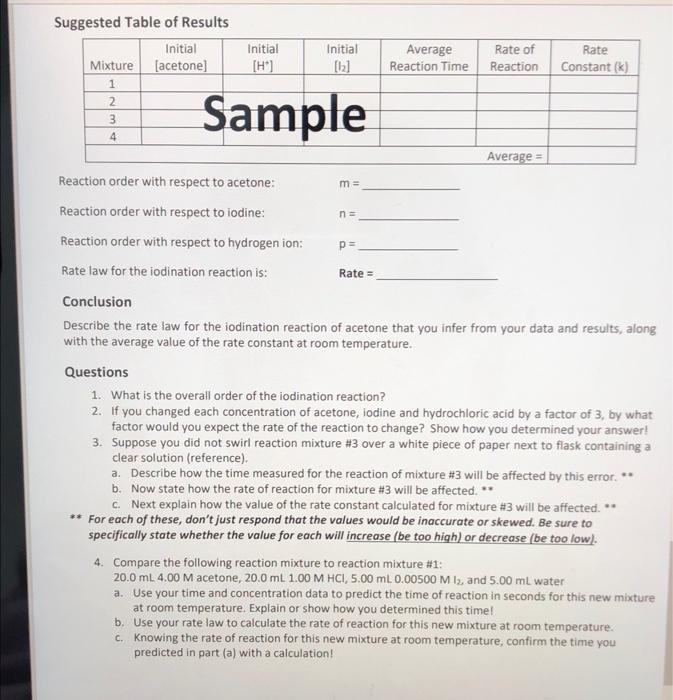
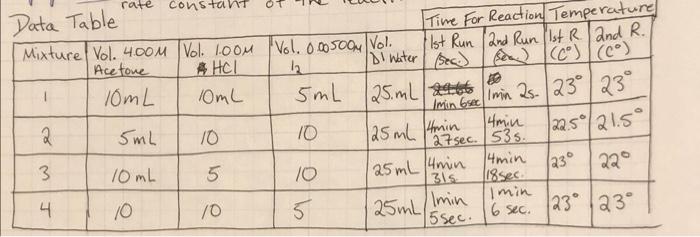
Mixture Rate of Reaction Rate Constant Constant (k) Initial Initial Average [H] [12] Reaction Time (sec) 0.2 0.00005 64 7.81x10-6 3.57x10-6 0.001 0.2 280 3 0.1 0.001 264.5 63 3.78*10-6 7.94x106 Average = 4 0.0005 0.2 rate=[Acetone] * [te1] [127 rate [Acetone] [HC174 [12] K- - z Suggested Table of Results Initial Mixture (acetone) Initial [H] Initial [12] Average Reaction Time Rate of Reaction Rate Constant (k) Nm 1 2 3 4 Sample m= na Average = Reaction order with respect to acetone: Reaction order with respect to iodine: Reaction order with respect to hydrogen ion: Rate law for the iodination reaction is: Conclusion Describe the rate law for the iodination reaction of acetone that you infer from your data and results, along with the average value of the rate constant at room temperature. p= Rate = Questions 1. What is the overall order of the iodination reaction? 2. If you changed each concentration of acetone, iodine and hydrochloric acid by a factor of 3, by what factor would you expect the rate of the reaction to change? Show how you determined your answer! 3. Suppose you did not swirl reaction mixture #3 over a white piece of paper next to flask containing a clear solution (reference). a. Describe how the time measured for the reaction of mixture #3 will be affected by this error. ** b. Now state how the rate of reaction for mixture #3 will be affected. ** c. Next explain how the value of the rate constant calculated for mixture #3 will be affected. * ** For each of these, don't just respond that the values would be inaccurate or skewed. Be sure to specifically state whether the value for each will increase (be too high) or decrease be too low). 4. Compare the following reaction mixture to reaction mixture #1: 20.0 ml. 4.00 M acetone, 20.0 ml 1.00 M HCI, 5.00 ml. 0.00500 M 13, and 5.00 ml water a. Use your time and concentration data to predict the time of reaction in seconds for this new mixture at room temperature. Explain or show how you determined this time! b. Use your rate law to calculate the rate of reaction for this new mixture at room temperature. c. Knowing the rate of reaction for this new mixture at room temperature, confirm the time you predicted in part (a) with a calculation! rate constan Data Table Time For Reaction Temperature Mixture Vol. 4.00M Vol. 1oom Vol. 0.005004 Vol. 1st Run 2nd Run (1st Rand R. Acetone 4 HCl 2 Di Water (sec) se (c) (6) 10mL 10mL 5mL 25 mL zat 2 4min 5mL 10 10 |22.5/21.5 27 sec. 535 3. 4min 10mL 5 10 22 31s. 118sec. min 4 10 10 Imin 5 6 sec. 123 23 15 min Grecimin 25. 23 23 125 ml 4min 125 mL 4min 230 4 125 mL s sec. Mixture Rate of Reaction Rate Constant Constant (k) Initial Initial Average [H] [12] Reaction Time (sec) 0.2 0.00005 64 7.81x10-6 3.57x10-6 0.001 0.2 280 3 0.1 0.001 264.5 63 3.78*10-6 7.94x106 Average = 4 0.0005 0.2 rate=[Acetone] * [te1] [127 rate [Acetone] [HC174 [12] K- - z Suggested Table of Results Initial Mixture (acetone) Initial [H] Initial [12] Average Reaction Time Rate of Reaction Rate Constant (k) Nm 1 2 3 4 Sample m= na Average = Reaction order with respect to acetone: Reaction order with respect to iodine: Reaction order with respect to hydrogen ion: Rate law for the iodination reaction is: Conclusion Describe the rate law for the iodination reaction of acetone that you infer from your data and results, along with the average value of the rate constant at room temperature. p= Rate = Questions 1. What is the overall order of the iodination reaction? 2. If you changed each concentration of acetone, iodine and hydrochloric acid by a factor of 3, by what factor would you expect the rate of the reaction to change? Show how you determined your answer! 3. Suppose you did not swirl reaction mixture #3 over a white piece of paper next to flask containing a clear solution (reference). a. Describe how the time measured for the reaction of mixture #3 will be affected by this error. ** b. Now state how the rate of reaction for mixture #3 will be affected. ** c. Next explain how the value of the rate constant calculated for mixture #3 will be affected. * ** For each of these, don't just respond that the values would be inaccurate or skewed. Be sure to specifically state whether the value for each will increase (be too high) or decrease be too low). 4. Compare the following reaction mixture to reaction mixture #1: 20.0 ml. 4.00 M acetone, 20.0 ml 1.00 M HCI, 5.00 ml. 0.00500 M 13, and 5.00 ml water a. Use your time and concentration data to predict the time of reaction in seconds for this new mixture at room temperature. Explain or show how you determined this time! b. Use your rate law to calculate the rate of reaction for this new mixture at room temperature. c. Knowing the rate of reaction for this new mixture at room temperature, confirm the time you predicted in part (a) with a calculation! rate constan Data Table Time For Reaction Temperature Mixture Vol. 4.00M Vol. 1oom Vol. 0.005004 Vol. 1st Run 2nd Run (1st Rand R. Acetone 4 HCl 2 Di Water (sec) se (c) (6) 10mL 10mL 5mL 25 mL zat 2 4min 5mL 10 10 |22.5/21.5 27 sec. 535 3. 4min 10mL 5 10 22 31s. 118sec. min 4 10 10 Imin 5 6 sec. 123 23 15 min Grecimin 25. 23 23 125 ml 4min 125 mL 4min 230 4 125 mL s sec
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


