Question: 2 A (aq) + 3 B (aq) Right arrow 4 C (s) + 5 D (aq) In the first 181 s of the given reaction,
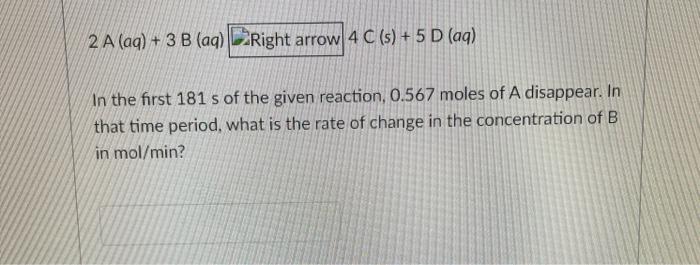
2 A (aq) + 3 B (aq) Right arrow 4 C (s) + 5 D (aq) In the first 181 s of the given reaction, 0.567 moles of A disappear. In that time period, what is the rate of change in the concentration of B in mol/min
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


