Question: 2. a) Components A and B in a solution are separated by the fractional distillation method. Given the boiling points component A and B are
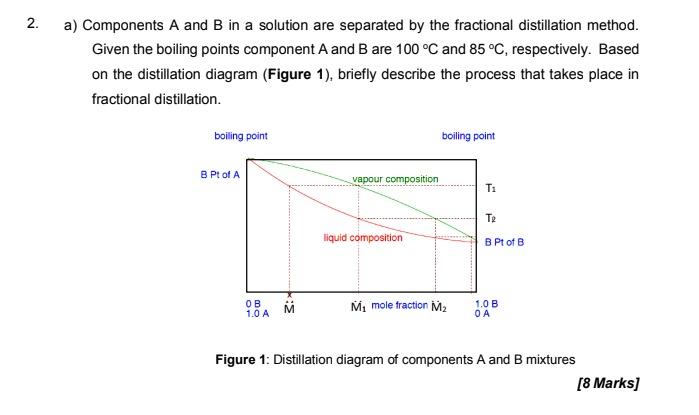
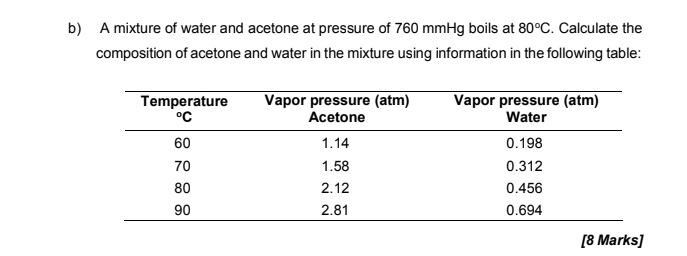
2. a) Components A and B in a solution are separated by the fractional distillation method. Given the boiling points component A and B are 100 C and 85 C, respectively. Based on the distillation diagram (Figure 1), briefly describe the process that takes place in fractional distillation. boiling point boiling point B Pt of A vapour composition T: Te liquid composition B Pt of B OB 1.0 A M mole fraction M2 1.0 B Figure 1: Distillation diagram of components A and B mixtures [8 Marks] b) A mixture of water and acetone at pressure of 760 mmHg boils at 80C. Calculate the composition of acetone and water in the mixture using information in the following table: Temperature C 60 70 80 90 Vapor pressure (atm) Acetone 1.14 1.58 2.12 2.81 Vapor pressure (atm) Water 0.198 0.312 0.456 0.694 [8 Marks]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


