Question: 2. A diamond cubic crystal structure is shown below (left). The image on the right shows the same structure from the perspective of the y-z
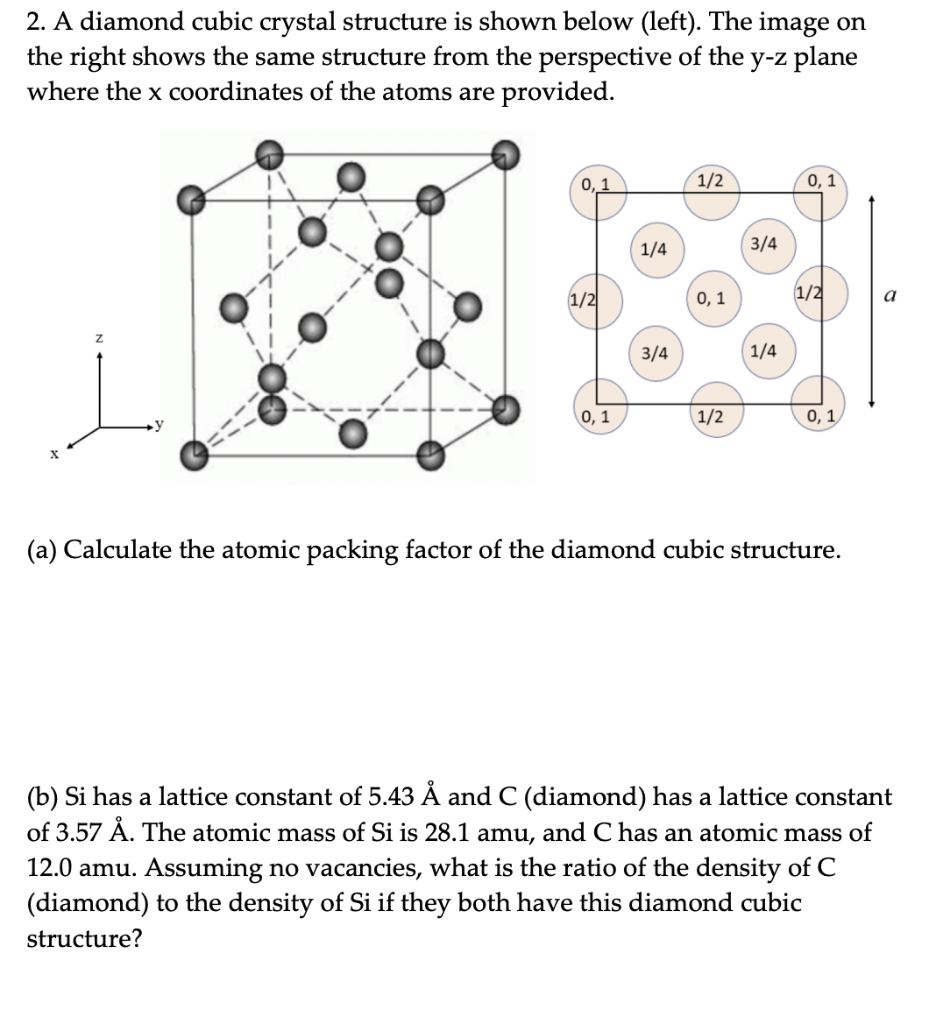
2. A diamond cubic crystal structure is shown below (left). The image on the right shows the same structure from the perspective of the y-z plane where the x coordinates of the atoms are provided. 0,1 1/2 0,1 1/4 3/4 1/2 0,1 1/2 a 3/4 1/4 0,1 1/2 0,1 (a) Calculate the atomic packing factor of the diamond cubic structure. a (b) Si has a lattice constant of 5.43 and C (diamond) has a lattice constant of 3.57 . The atomic mass of Si is 28.1 amu, and C has an atomic mass of 12.0 amu. Assuming no vacancies, what is the ratio of the density of C (diamond) to the density of Si if they both have this diamond cubic structure
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


