Question: (2) (a) Find two different ways to linearize the equation r=Az/(B+z) B are adjustable constants. The reaction rate is r, and the concentration is z.
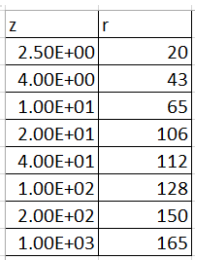
(2) (a) Find two different ways to linearize the equation r=Az/(B+z) B are adjustable constants. The reaction rate is r, and the concentration is z. Use both linearizations to fit the data to the right using perpendicular offsets. Compare the values of the constants for the two linearizations and the quality Q2 of the fits. (b) Use the solver on Excel to find a numerical solution for fitting the nonlinear equation to the data using vertical offsets. You will need a reasonable first guess for the solver in order to get the numerical method to converge. Use one of your results in part (a) for this guess. Compare this result with the two results in part (a). \begin{tabular}{|l|r|} \hlinez & r \\ \hline 2.50E+00 & 20 \\ \hline 4.00E+00 & 43 \\ \hline 1.00E+01 & 65 \\ \hline 2.00E+01 & 106 \\ \hline 4.00E+01 & 112 \\ \hline 1.00E+02 & 128 \\ \hline 2.00E+02 & 150 \\ \hline 1.00E+03 & 165 \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


