Question: 2. A high molecular weight hydrocarbon is decomposed into small molecules thermally in a 0.1 L BMR. The gas phase reaction stoichiometry can be assumed
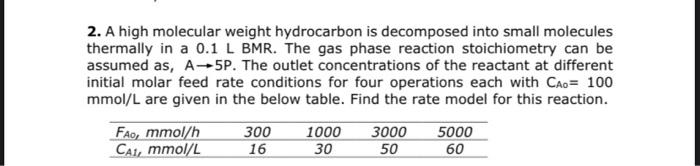
2. A high molecular weight hydrocarbon is decomposed into small molecules thermally in a 0.1 L BMR. The gas phase reaction stoichiometry can be assumed as, A-5P. The outlet concentrations of the reactant at different initial molar feed rate conditions for four operations each with Cao= 100 mmol/L are given in the below table. Find the rate model for this reaction. Fao, mmol/h 300 1000 3000 5000 Cai, mmol/L 16 50 60 30
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


