Question: Q2 (35 p): The high molecular weight reactant A is thermally decomposed into low molecular weight products by a homogeneous gas-phase reaction of A 4P
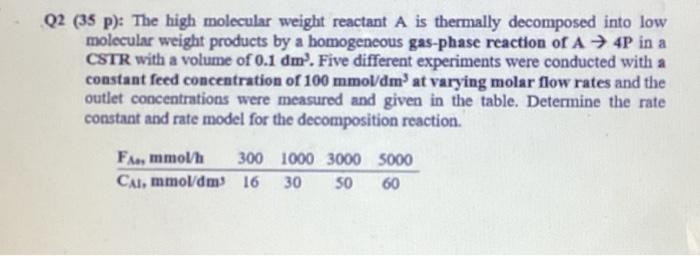
Q2 (35 p): The high molecular weight reactant A is thermally decomposed into low molecular weight products by a homogeneous gas-phase reaction of A 4P in a CSTR with a volume of 0.1dm3. Five different experiments were conducted with a constant feed concentration of 100mmoldmm3 at varying molar flow rates and the outlet concentrations were measured and given in the table. Determine the rate constant and rate model for the decomposition reaction. Q2 (35 p): The high molecular weight reactant A is thermally decomposed into low molecular weight products by a homogeneous gas-phase reaction of A 4P in a CSTR with a volume of 0.1dm3. Five different experiments were conducted with a constant feed concentration of 100mmoldmm3 at varying molar flow rates and the outlet concentrations were measured and given in the table. Determine the rate constant and rate model for the decomposition reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


