Question: 2. A liquid phase reaction below takes place in a batch reactor. The initial concentration of ethylene oxide and water, after mixing in the reactor,
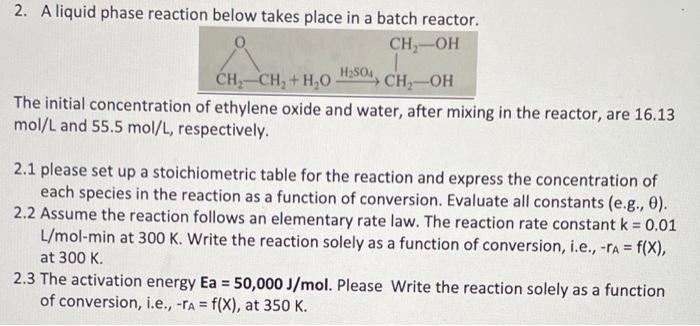
2. A liquid phase reaction below takes place in a batch reactor. The initial concentration of ethylene oxide and water, after mixing in the reactor, are 16.13 mol/L and 55.5mol/L, respectively. 2.1 please set up a stoichiometric table for the reaction and express the concentration of each species in the reaction as a function of conversion. Evaluate all constants (e.g., ). 2.2 Assume the reaction follows an elementary rate law. The reaction rate constant k=0.01 L/molmin at 300K. Write the reaction solely as a function of conversion, i.e., rA=f(X), at 300K. 2.3 The activation energy Ea=50,000J/mol. Please Write the reaction solely as a function of conversion, i.e., rA=f(X), at 350K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


