Question: 2. A solution is made by dissolving 25gNaCl in cnough water to make 1.0L, solution. Assuming the density of the solution is 1.0g.cm3. Calculate the
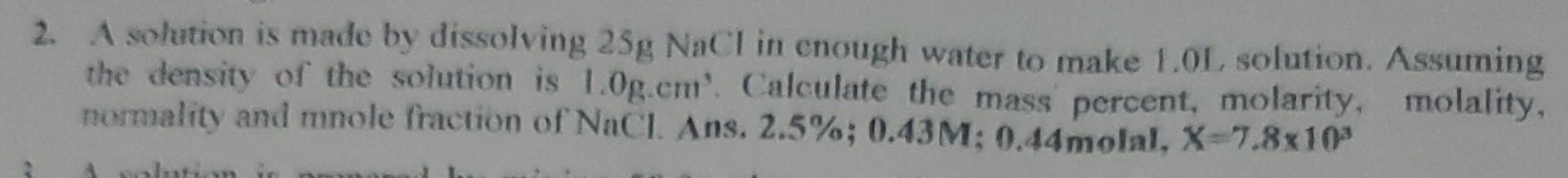
2. A solution is made by dissolving 25gNaCl in cnough water to make 1.0L, solution. Assuming the density of the solution is 1.0g.cm3. Calculate the mass percent, molarity, molality, normality and mnole fraction of NaCl. Ans. 2.5%;0.43M;0.44molal,X=7.8103
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


