Question: Enter your answer in the provided box. A solution is prepared by dissolving 416g of sucrose (C12H22O11) in 599g of water. What pressure of this
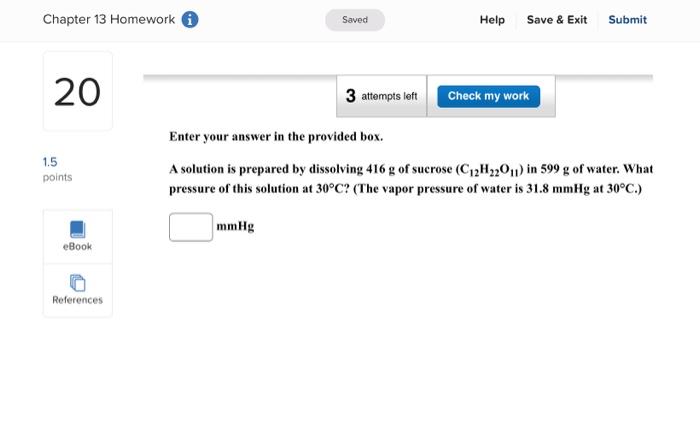
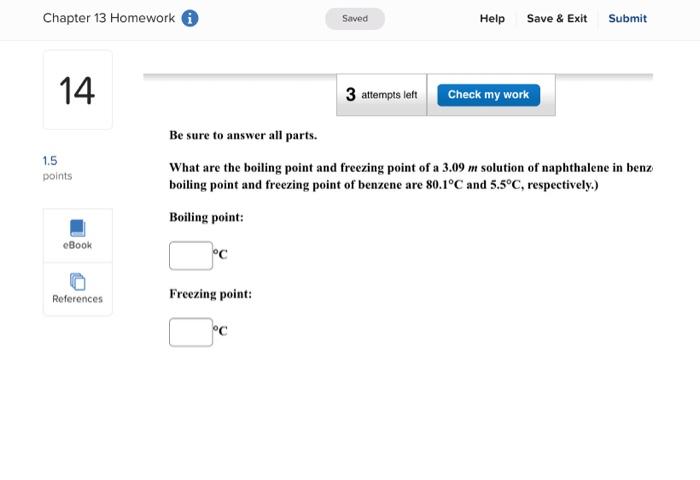
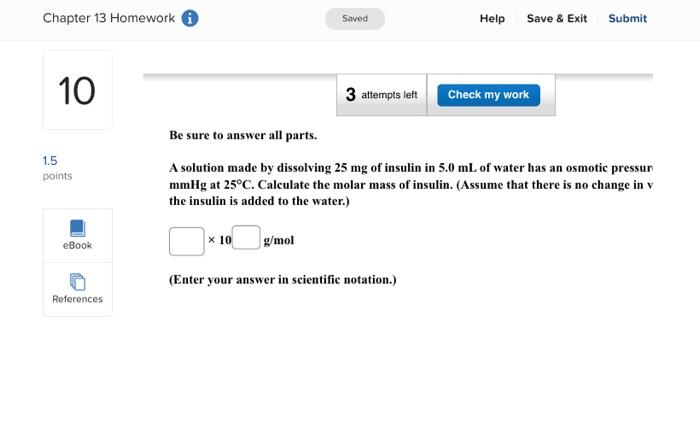
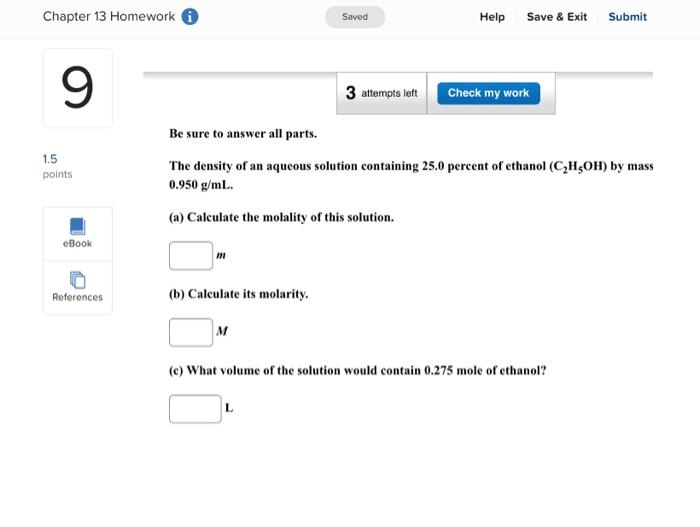
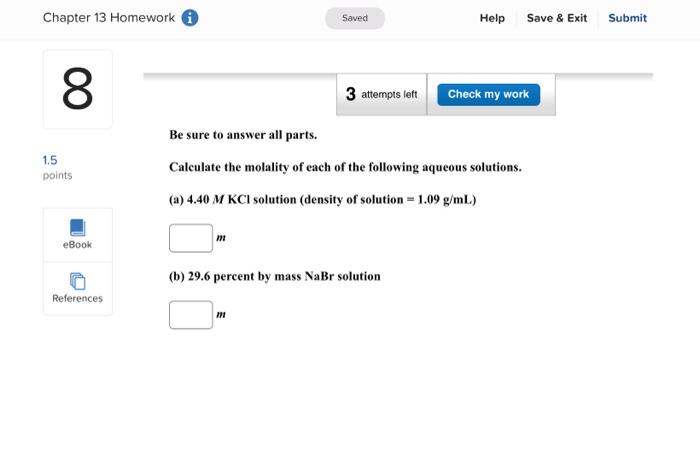
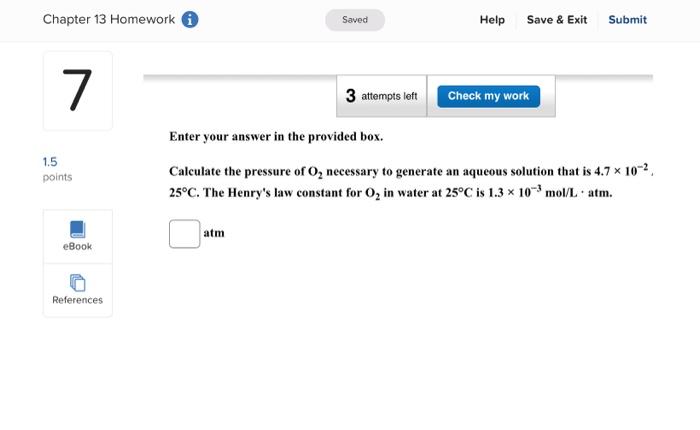
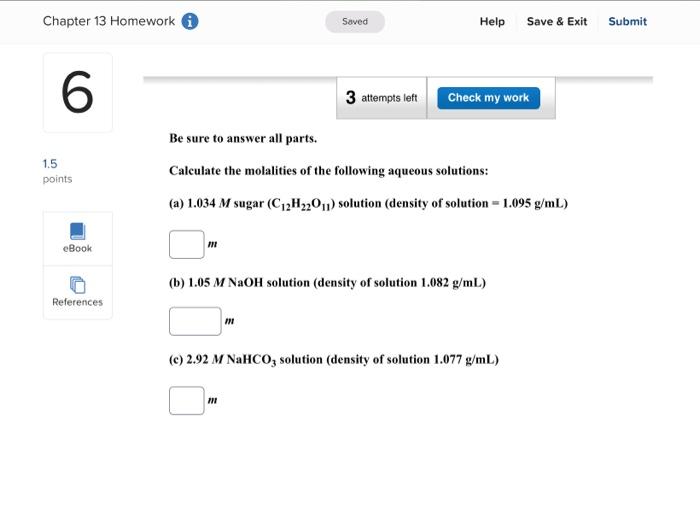
Enter your answer in the provided box. A solution is prepared by dissolving 416g of sucrose (C12H22O11) in 599g of water. What pressure of this solution at 30C ? (The vapor pressure of water is 31.8mmHg at 30C.) mmHg Be sure to answer all parts. What are the boiling point and freezing point of a 3.09m solution of naphthalene in benz boiling point and freezing point of benzene are 80.1C and 5.5C, respectively.) Boiling point: C Freezing point: 3C Be sure to answer all parts. A solution made by dissolving 25mg of insulin in 5.0mL of water has an osmotic pressur mmHg at 25C. Calculate the molar mass of insulin. (Assume that there is no change in v the insulin is added to the water.) 10g/mol (Enter your answer in scientific notation.) Be sure to answer all parts. The density of an aqueous solution containing 25.0 percent of ethanol (C2H5OH) by mass 0.950g/mL. (a) Calculate the molality of this solution. m (b) Calculate its molarity. M (c) What volume of the solution would contain 0.275 mole of ethanol? L Be sure to answer all parts. Calculate the molality of each of the following aqueous solutions. (a) 4.40MKCl solution (density of solution =1.09g/mL ) m (b) 29.6 percent by mass NaBr solution m Enter your answer in the provided box. Calculate the pressure of O2 necessary to generate an aqueous solution that is 4.7102, 25C. The Henry's law constant for O2 in water at 25C is 1.3103mol/Latm. atm Be sure to answer all parts. Calculate the molalities of the following aqueous solutions: (a) 1.034M sugar (C12H22O11) solution (density of solution =1.095g/mL ) m (b) 1.05MNaOH solution (density of solution 1.082g/mL ) m (c) 2.92MNaHCO solution (density of solution 1.077g/mL ) m
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


