Question: 2. An aqueous caustic stream mixture containing 20% (by weight) sodium hydroxide (NaOH) is fed to an evaporator to remove 50% of the water in

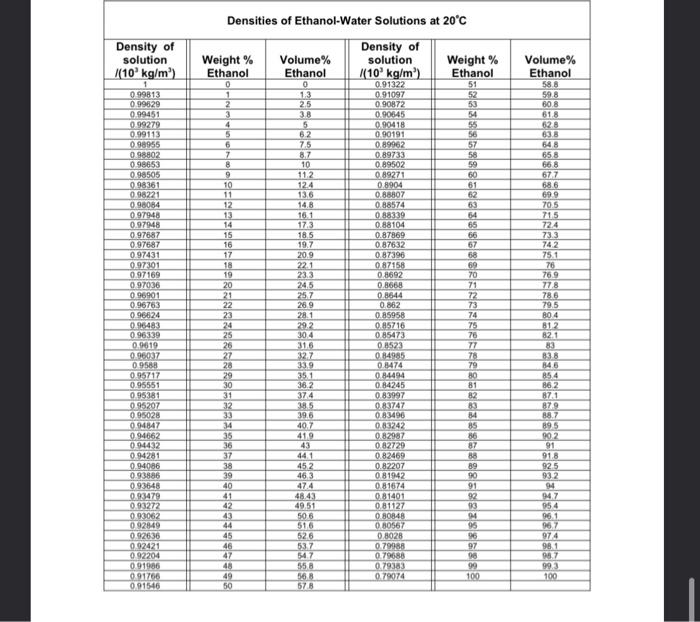
2. An aqueous caustic stream mixture containing 20% (by weight) sodium hydroxide (NaOH) is fed to an evaporator to remove 50% of the water in the feed stream. Draw and label a PFD of the process. Using a Degree of Freedom analysis show that there is insufficient information to allow you to balance the PFD. If you assume a basis of a feed stream of 100 kg/h can you now proceed? If so calculate all unknown stream flows and compositions. Express the flow rate and composition of the concentrated caustic stream in molar quantities. 3. A gas stream containing 40 mole % O2, 40 mole % Hz and the balance is water, is dried by cooling the gas stream and condensing out the water. If 10 th of the gas is to be processed what is the condensation rate assuming all of the water in the feed is condensed and what is the composition of the dry gas. 4. A batch of soya beans contains 18% oil and 10% water by weight. It is treated with pure hexane to give a solution containing 25% oil and 75% hexane and a residue containing 0.5% oil and 29% hexane. What weight of soya beans is required to yield 1000 kg of the hexane/oil solution? 5. A certain soap contains 60% moisture on a wet basis in the raw state. Before it can be pressed into bars of soap the moisture content must be reduced by evaporation to 20% on a wet basis. How many 125 g bars of soap can be produced from 1 tonne of original raw soap? 6. An aqueous solution of 20 mol% NaOH is to be prepared on a continuous basis by mixing pure solid NaOH with a pure stream of deionised water. What is the addition rate of each stream required to produce 0.5 th of the product stream? 7. Two liquid streams are flowing at constant rates into a blender. Benzene (p = 879 kg/m) flows at a measured rate of 20,0 L/min, and the other is Toluene (p = 866 kg/m). The blended mixture enters a storage tank (inner diameter = 5,5 m) equipped with a sight gauge. During an interval in which no liquid leaves the storage tank, the liquid level in the tank is observed to increase by 0.15 meters over a one-hour period. Calculate the flow rate of toluene into the blender (L/min) and the composition of the tank contents (wt% benzene). 8. A liquid mixture of Benzene (CH) and n-Decane (CtoHa) (% = 0.55 kg/kg) is to be partially evaporated in a batch still. The vapour is condensed to produce a liquid mixture containing 85% Benzene and the residual liquid in the still contains 10.6% Benzene by mass. 0 Calculate the average molecular mass of the initial charge to the still C) | 20 kmol of the mixture are initially charged to the still, determine the masses of the final product streams. (T) Express the compositions of the final streams as mole fractions. Density of solution (10 kg/m) Volume% Ethanol Densities of Ethanol-Water Solutions at 20C Density of Weight % Volume% solution Weight % Ethanol Ethanol (10 kg/m) Ethanol 0 0 0.91322 51 1 13 0.91097 52 2 2.5 0.90872 53 3 38 0.90645 4 5 0.90418 55 5 62 0.90191 56 6 75 0.89962 57 7 8.7 0.89733 58 8 10 0.89502 59 9 112 0.89271 60 10 124 0.8904 61 11 13.6 0.88807 62 12 14.8 0.88574 63 13 16.1 0.88339 64 14 17.3 0.88104 65 15 185 0.87869 66 16 19.7 0.87632 67 17 20.9 0.87396 68 18 221 0.87158 69 19 23.3 0.8692 70 20 24,5 0.8668 71 21 257 0.8644 72 22 28.9 0.862 73 23 28.1 0.85958 74 24 29.2 0.85716 75 25 304 0.85473 76 26 31.5 0.1523 77 27 32.7 0.84985 78 28 339 0.8474 79 29 351 0.84494 80 30 36.2 0.84245 81 31 374 0.83997 82 32 38.5 0.83747 33 39.6 0.83496 84 34 40.7 0.83242 85 35 41.9 0.82987 86 36 0.82729 87 37 441 0.82469 88 38 452 0.82207 89 39 463 081942 90 40 47.4 081674 91 41 4843 0.81401 92 42 49.51 081127 33 43 505 080848 94 44 51.6 080567 95 45 526 08028 96 46 537 0.79988 97 47 547 0.79688 98 48 958 0.79383 99 49 36,8 0.790974 100 50 578 0.99813 0.99629 0.99451 099279 0.99113 098955 098802 0.98653 0.98505 098361 098221 9.98084 0.97948 0.979448 9.97687 0.97687 0.97431 0.97301 0.97169 0.97036 096901 0.96763 0.96624 0.95483 0.96339 0.9619 0.00037 09588 0.95717 0.95551 0.95381 0.95207 0.95028 0.94347 0.94662 0.94432 0.94281 0.94086 0.93886 093548 0.93479 0.93272 0.93062 092849 092636 0.92421 092204 091986 0.91766 0.91546 598 80.8 618 628 638 548 658 668 677 68.6 699 705 715 724 73.3 742 75.1 76 76.9 778 786 79.5 B04 812 82.1 838 84.6 85.4 862 871 879 88.7 895 90.2 91 91.8 925 932 94 947 954 96.1 967 974 98.1 987 993 100
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


