Question: 2 and 5 are correct but I dont know which one is the incorrect one Select one incorrect statement. When the phase diagram of a
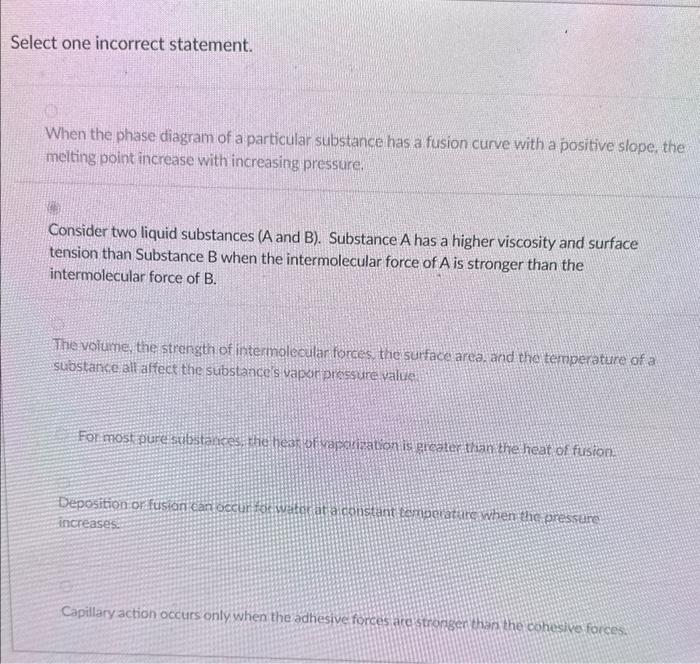
Select one incorrect statement. When the phase diagram of a particular substance has a fusion curve with a positive slope, the melting point increase with increasing pressure. Consider two liquid substances (A and B). Substance A has a higher viscosity and surface tension than Substance B when the intermolecular force of A is stronger than the intermolecular force of B. The voiume the strength of intermalecular forces, the surface area, and the temperature of a substanceiall affect the substance s vapor pirsesure value Capillary action occurs only when the adhesive forces arcistronser than he cohesive forces
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


