Question: 2) (application) Consider the processes (A)-(D), as plotted on a PV diagram. Each of these processes takes the same ideal gas from an identical
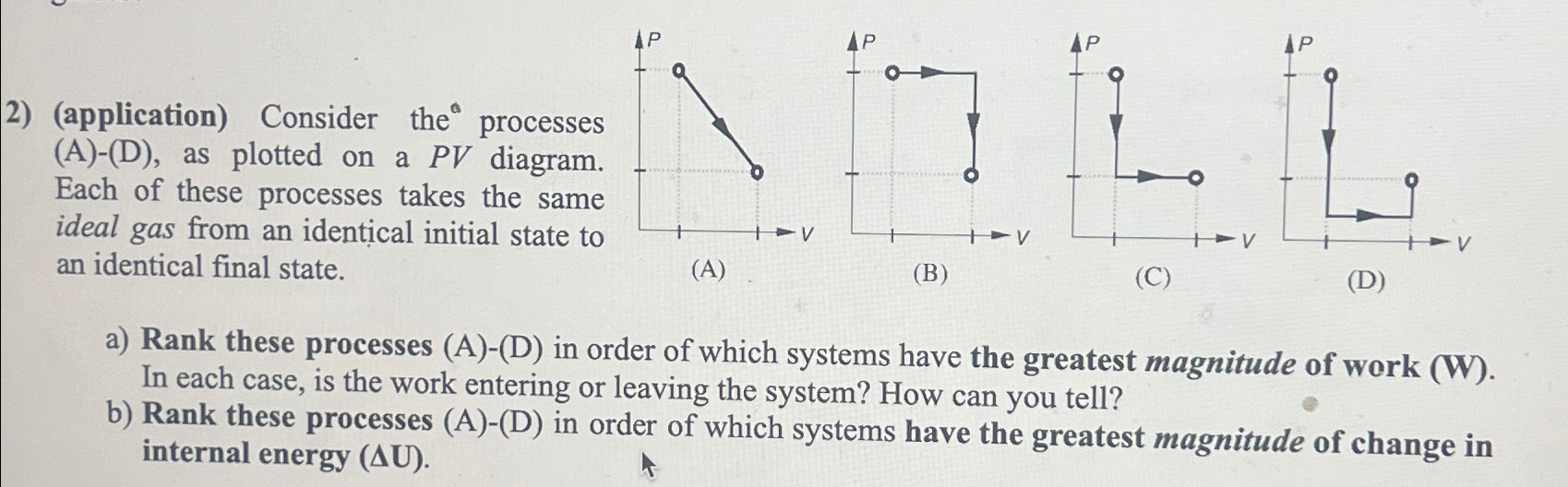
2) (application) Consider the processes (A)-(D), as plotted on a PV diagram. Each of these processes takes the same ideal gas from an identical initial state to an identical final state. LOLL (A) (B) (C) (D) a) Rank these processes (A)-(D) in order of which systems have the greatest magnitude of work (W). In each case, is the work entering or leaving the system? How can you tell? b) Rank these processes (A)-(D) in order of which systems have the greatest magnitude of change in internal energy (AU).
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


